New ways to use biomass
Tungsten carbide as catalyst for cost-effective conversion of cellulose into industrially useful carbon compounds
Alternatives to fossil fuels and natural gas as carbon sources and fuel are in demand. Biomass could play a more significant part in the future. Researchers in the USA and China have now developed a new catalyst that directly converts cellulose, the most common form of biomass, into ethylene glycol, an important intermediate product for chemical industry. As reported in the journal Angewandte Chemie, the catalyst is made of tungsten carbide and nickel on a carbon support.
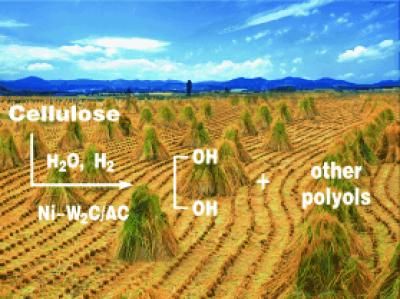
The expense of using precious-metal catalysts is avoided in the high-yielding conversion of cellulose to ethylene glycol (see picture; AC=activated carbon). This process occurs in up to 29 percent yield over a tungsten carbide catalyst, and in up to 61 percent yield when the catalyst is promoted with a small amount of nickel. An attractive feature of this reaction is the low yields of other polyols with respect to ethylene glycol.
Wiley-VCH 2008
Currently, biomass is mainly used in the form of starch, which is degraded to make sugars and then fermented to make ethanol. It would be cheaper to use cellulose, which is the main component of plant cell walls and thus the most plentiful organic compound on Earth. In contrast to starch from corn and grain, cellulose is not a food, so there would be no competition between its use as food or as raw material and fuel. At the moment, cellulose is mainly processed by fermentation. However, splitting cellulose into its individual sugar components, which can then be fermented, is a slow and cost-intensive process. The direct conversion of cellulose into useful organic compounds is thus an attractive alternative.
Initial reactions using various noble-metal catalysts have been developed. Their disadvantage is that large amounts of expensive metal are needed to break down the cellulose. On an industrial scale, these processes are thus not economical. A less costly and more effective catalyst is needed.
A team led by Tao Zhang at the Dalian Institute of Chemical Physics (China) and Jingguang G. Chen at the University of Delaware (Newark, USA) has now developed just such a system. The catalyst is made of tungsten carbide deposited on a carbon support. Small amounts of nickel improve the efficiency and selectivity of the catalyst system: a synergetic effect between the nickel and tungsten carbide not only allows 100% conversion of cellulose, but also increases the proportion of ethylene glycol in the resulting mixture of polyalcohols to an amazing 61%. Ethylene glycol is an important intermediate in the chemical industry. For example, in the plastics industry it is needed for the production of polyester fibers and resins, and in the automobile industry it is used as antifreeze.
Original publication: Jingguang G. Chen et al.; "Direct Catalytic Conversion of Cellulose into Ethylene Glycol Using Nickel-Promoted Tungsten Carbide Catalysts"; Angewandte Chemie International Edition 2008.
Most read news
Topics
Organizations
Related link
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Hard_and_soft_drugs

Printing nanomaterials with plasma
Salamander_(metallurgy)
Nordihydroguaiaretic_acid

Scientists develop a method to turn hazardous acidic industrial wastewater into valuable resources - The process reducing the volume of wastewater by 90%