PainCeptor Pharma announces positive clinical results that validate ASICs as novel targets for human pain
PainCeptor Pharma announced that PPC-5650, a potent ASIC1a antagonist, reduces thermal and mechanical hyperalgesia in a human inflammatory pain model, thereby validating ASICs as novel molecular targets for the treatment of pain.
PPC-5650, an investigational drug, is a selective and potent peripherally-acting ASIC1a antagonist being developed for the treatment of acute and chronic inflammatory pain. The randomized, double-blind, placebo-controlled study tested the efficacy of a single intradermal dose of PPC-5650 on primary hyperalgesia in 15 healthy male subjects in a UVB inflammatory pain model. The trial was conducted at the Medical University of Vienna, Austria, with Dr. Burkhard Gustorff, MD, DEAA as principal investigator. Dr Gustorff states "It is very exciting to report on these positive, first time in human results for a novel class of compounds for the treatment of pain."
The results from the study demonstrate that PPC-5650 exerts a significant (p(less than)0.05) treatment effect on heat pain tolerance threshold and mechanical pain sensitivity.
Most read news
Other news from the department research and development

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
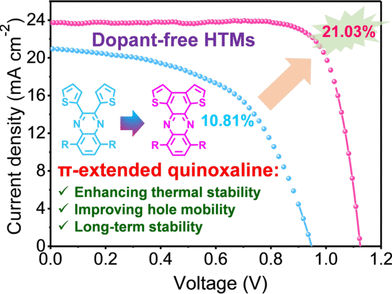
Purely Organic Hole Transporter - Dopant-free, humidity-stable organic layers give perovskite solar cells 21% efficiency

PielColor Uruguay S.A. - Paso de la Arena, Montevideo, Uruguay

Matuszak-Hygiene GmbH & Co. KG - Sprendlingen, Germany

Trigona OHG - Wiesbaden, Germany



























































