Wacker Expands Biologics Operation in Jena, Germany
Wacker is strengthening its biopharmaceutical operations in Jena, Germany. The Munich-based chemical Group is investing around EUR15 million in this undertaking, which involves the GMP expansion of its Jena production facility and the construction of a new building there for process development and quality control. Through Wacker Biotech GmbH, Wacker 'sJena-based subsidiary, the Group intends to secure further growth in biologics (pharmaceutical proteins) over the next few years and to strengthen its position in this market.
"The new facilities will enable us to greatly accelerate our growth in the attractive biologics market," states Dr. Gerhard Schmid, president of WACKER FINE CHEMICALS, the Group's biotech and fine chemicals division.
Wacker's investment involves two expansion projects at its Jena site, which is on the Beutenberg Campus (where 1,500 scientists conduct research). The first project will double the manufacturing space at the existing GMP facility, partly by adding a completely new purification station to help ease bottlenecks. This station will also comply with GMP pharmaceutical manufacturing standards, in accordance with FDA (US Food and Drug Administration) and EMEA (European Medicines Evaluation Agency) regulations. The expansion will give Wacker Biotech the capacity customers need for biologics that are almost ready for market supply. The new facility is scheduled to come on stream at the end of 2009.
The second project concerns a new process-development and quality-control building. The focus here is on a proprietary E. coli-based proteine secretion technology developed by Wacker. Wacker Biotech is expanding its process-development facilities to meet increasing customer demand. Wacker Biotech's quality control labs are being expanded, as well, to enable the company to optimally satisfy ever-growing demand for detailed product and process characterization. The new building is scheduled for completion in late 2008.
Other news from the department manufacturing

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Start-up for Mobile High-Performance Battery Systems Raises 33 Million U.S. Dollars - The technology offers mobile power supply from renewable sources that is entirely disconnected from the power grid

Elemental Analysis of Sodium Ion Battery Cathode Materials by Agilent ICP-OES

Fluorinated plastics with an expiry date - The fluorine can be recovered from the degraded material to be reincorporated into all kinds of chemicals
Pierre_de_Jaumont

New clues help explain why PFAS chemicals resist remediation - Work suggests new avenues for cleaning up these 'forever chemicals'

Mikroskopie-Zubehör | Microscopy accessories | AHF analysentechnik
For a Better Contrast - Rare earth orthoferrite LnFeO3 nanoparticles for bioimaging
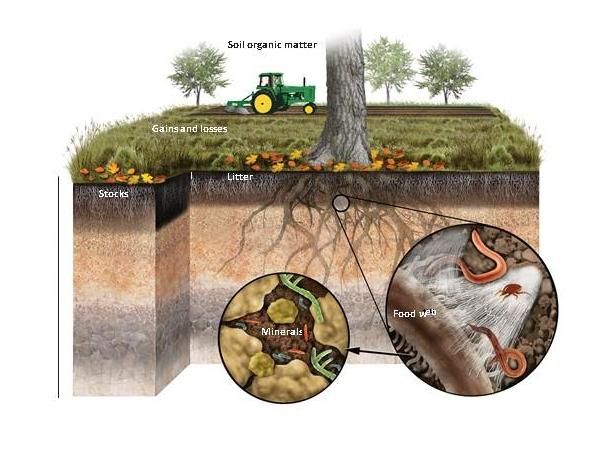
Huge carbon sink in soil minerals
Symbiotic CO₂ Sequestration - Bioengineered microbial community working together to store carbon
Category:Atmospheric_chemistry