Genmab Initiates Second Phase II Study With HuMax-CD4
Genmab A/S (CSE: GEN and Neuer Markt: GE9D) announced today that it has initiated a Phase II clinical trial with its fully human antibody HuMax-CD4(TM) to treat patients with severe psoriasis. This multi-center study will include four different dose levels plus a placebo arm and it is designed to provide safety and efficacy data, as well as dose finding information.
HuMax-CD4 Phase I/II results were presented at the American College of Rheumatology meeting in Philadelphia in November and showed that the fully human antibody was well tolerated and that patients' CD4 counts remained stable in response to the treatment. In this single dose study, the four highest dose cohorts, 0.5, 1, 2 and 4 mg/kg, fifty percent of the treated patients achieved favorable responses to the antibody, as measured by objective criteria defined by the American College of Rheumatology and routinely used by companies seeking regulatory approval of arthritis products. Eight of 16 achieved ACR 20, one of those achieved ACR 50, and two of the group achieved ACR 70.
"Genmab has achieved another development milestone with the initiation of this second Phase II study with HuMax-CD4," said Lisa. N. Drakeman, Ph.D., Chief Executive Officer "Current treatments can be extremely toxic to patients and new therapies are urgently needed for the millions of people who suffer from psoriasis."
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
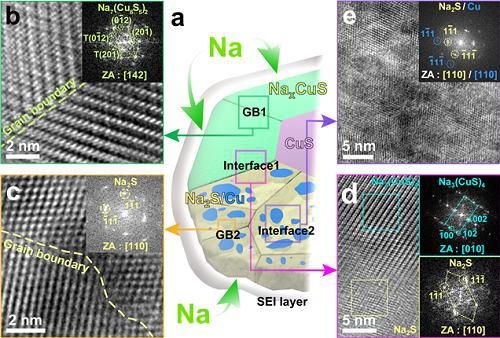
High-performance sodium ion batteries using copper sulfide
LANXESS inaugurates new formalin plant in Krefeld-Uerdingen - EUR 18 million investment in new production operations

LANXESS to enlarge its menthol facility in Krefeld
Electrification of Commersial Vehicles
Bayer will extend polycarbonate sheet business - Agreement for acquisition in the USA
Insights on Theion’s Lithium Sulfur Battery Technology



























































