Radical research results on the oxidation of vitamin E
Recent research results have challenged conventional understanding of the Oxidation of the "radical scavenger", vitamin E. Cutting-edge analysis methods have revealed that the intermediates commonly believed to be involved in the process do not occur. This surprising finding has been systematically documented and published as part of a project supported by the Austrian Science Fund FWF. The new findings are also extremely important for a follow-up project that is focusing on the synthesis of "super antioxidants" based on a polymeric vitamin E.

Vitamin E: The interest in basic chemistry of a well-known molecule is blooming again.
Thomas Rosenau
Vitamin E is one of the most important naturally occurring antioxidants, and has become widely known as a "radical scavenger" and anti-ageing product. It is perhaps precisely this high profile that has caused scientific interest in vitamin E to focus on optimizing its usage, while aspects relating to the basic chemistry have received less attention. However, in their work to develop new potential applications, chemists are revisiting these basics - and are radically rewriting a great deal of accepted teaching as they do so.
Intermediate & End Result One such chemist is Prof. Thomas Rosenau from the Department of Chemistry at the University of Natural Resources and Applied Life Sciences in Vienna. During their work, he and his team closely analyzed the chemical processes involved in the one-electron oxidation of alpha-tocopherol (the main component of vitamin E), which conventional understanding dictates is proceeding via certain intermediates, known as C-centred radicals with an o-quinone methide (oQM) structure. However, the results produced by Prof. Rosenau's team entirely disprove this theory.
Prof. Rosenau explains: "In principle, oQMs have low stability and a short lifespan. This makes it extremely difficult to demonstrate their presence and investigate them. However, we succeeded in significantly improving the stability of oQMs and extending their lifespan to up to 20 minutes. This enabled us to monitor oQMs directly in our analysis work on reaction processes."
Because extreme caution should be exercised when refuting accepted teaching, Prof. Rosenau's team ensured that all analyses were founded on a solid basis. A number of independent analytical methods were employed to confirm the team's results. These included electron paramagnetic resonance spectroscopy in liquid and solid phases, electron spin resonance spectroscopy, mass spectroscopy, the isotopic labelling of special derivatives, investigation of reaction kinetics and the development of computer models.
If the Chemistry is Right ... Prof. Rosenau on the significance of his findings, which are being published in The Journal of Organic Chemistry and Chemistry - a European Journal: "At first glance, the question as to whether C-centred radicals with oQM structures are generated as a short-lived intermediate during the oxidation of alpha-tocopherol may seem like a question of purely academic interest. It is not! These radicals exhibit an extremely high reactivity and can therefore inflict major damage on cells. As alpha-tocopherol performs a whole range of physiological functions as a fat-soluble antioxidant and is present in a wide variety of medicines, healthcare products, foods and cosmetics, it is vitally important that we understand all the potentially harmful side effects that its degradation products can have on the health of patients and consumers."
But there is another reason why Prof. Rosenau's team chose to investigate the reactivity of alpha-tocopherol products. A second FWF project is looking at the synthesis of polytocopherols using a brand new reaction called spiropolymerisation, which is based on the understanding of the oxidation reaction. Polytocopherols are molecules where numerous tocopherol units are interconnected in chains or rings. oQMs are used to form these connections, while highly reactive radicals lead to crosslinking and breaks in the individual polymer chains, which would thereby make it much more difficult to synthesise polytocopherols. The scientific work of Prof. Rosenau demonstrates that progress in current scientific issues can sometimes mean revising what we think we know.
Original publication: T. Rosenau, E. Kloser, L. Gille, F. Mazzini & T. Netscher: Vitamin E Chemistry. Studies into Initial Oxidation Intermediates of alpha-Tocopherol: Disproving the Involvement of 5a-C-centered "Chromanol Methide" Radicals. J. Org. Chem. 2007, 72, 3268 - 3281; T. Rosenau, G. Ebner, A. Stanger, S. Perl, L. Nuri: From a Theoretical Concept to Biochemical Reactions: Strain Induced Bond Localization (SIBL) in Oxidation of Vitamin E. Chem. Eur. J. 2005, 11(1), 280-287
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.
Last viewed contents
Borealis re-assigns roles in Polyolefins Leadership Team
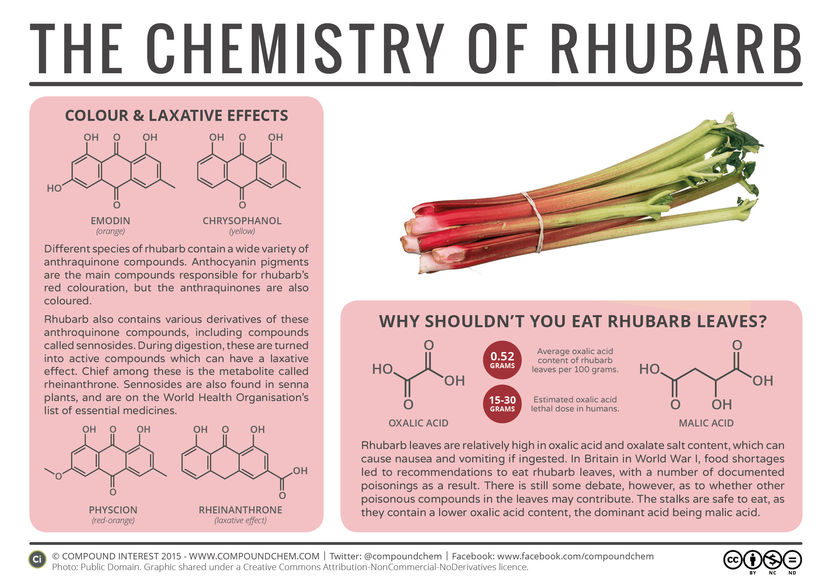
Why Shouldn’t You Eat Rhubarb Leaves?
Bayer MaterialScience LLC appoints Dirk Schapeler CEO of Artificial Muscle, Inc.

Facility Monitoring Systems Ltd - Great Malvern, United Kingdom
Azelis Deutschland wins Bluestar award for Market Development for silicones and other specialities
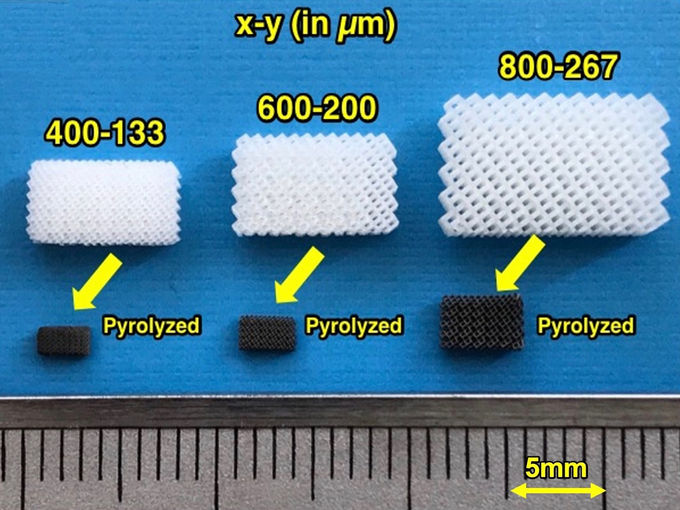
Micro-engineered electrodes could cut battery manufacturing costs - "As 3D printers gain increasing resolution, sodium ion batteries could eventually outperform lithium ion ones"

Full focus on electrolysis - Sunfire spins off its fuel cell business, Sunfire Fuel Cells





























































