Coming Soon: Protein Synthesis Without Amino Acids?
Polypeptide synthesis done differently: cobalt-catalyzed copolymerization of imines and CO
Advertisement
Usually, the synthesis of short protein chains (polypeptides) begins with the production of their components, the amino acids. But it can be done differently: In the journal Angewandte Chemie, Chinese researchers report a considerably more convenient method that is similar to olefin polymerization, which is used for the mass production of plastics such as polyethylene. The advantage of this reaction is that it uses inexpensive starting materials and would be ideal for industrial production.
Whether in the body or the factory, the backbone of polypeptide chains is usually formed by the linking of an amino group with the acid group of individual amino acids. Like pearls on a string, the amino acids then line up. However, to get to such a structure, it isn't absolutely necessary to start from amino acids. Imines, compounds with a carbon-nitrogen double bond, could be an ideal starting material-if it were possible to link them together in an alternating fashion with a carbon monoxide molecule (CO), like a pearl necklace made of two different alternating types of pearl. This long-envisioned process is modeled after the plastic production process known as Ziegler-Natta polymerization, which requires special metal catalysts. The heart of this process is a step called insertion, in which the next "pearl", or monomer, squeezes itself in between the metal atom and the growing chain.
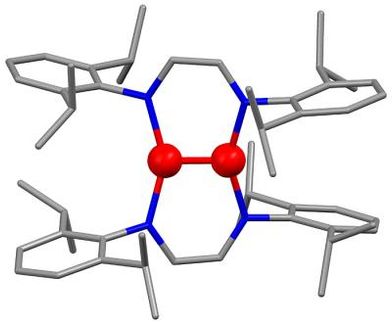
Until now, attempts to use this type of copolymerization for peptide synthesis have failed because of the lack of a suitable, effective, and continuously operating catalyst. Researchers led by Huailin Sun at Nankai University in China have now found a catalyst to do the job: a simple cobalt complex. The team was thus able to use this technique to synthesize polypeptides that have previously not been accessible by other means.
As a next step, the Chinese researchers want to include not just one, but a variety of imines into the same chain.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.