Pfizer Says Research and Development Head John LaMattina Will Retire This Year
Company to Focus on Accelerating Development and Commercialization of Strong Early- and Mid-Stage Pipeline
Pfizer said that the President of Pfizer Global Research and Development, Dr. John LaMattina, who made many important contributions to the Company's research division throughout his 30-year career, will retire from Pfizer by the end of this year. The company will begin a search both inside and outside the company for his successor, and Dr. LaMattina has agreed to remain during this period to ensure a smooth transition.
"Science is at the heart of Pfizer, where world-class scientists use cutting-edge technologies to find new cures for diseases that cut short far too many lives," said Jeff Kindler, Pfizer's Chairman and Chief Executive Officer. "Pfizer now has a significant array of early- and mid-stage product candidates across a range of important therapeutic areas, and John has made a critical contribution to building this foundation. With that in place, John felt it was the right time to retire as we look to the future and accelerate the development of our most promising compounds so that they will be ready for commercialization as rapidly as possible."
Pfizer's research now includes 249 total programs with new therapies in development for obesity, diabetes, rheumatoid arthritis, schizophrenia, oncology, liver disease, HIV and Alzheimer's disease, among others. To further enhance the productivity of the R&D organization, Pfizer announced a series of initiatives at its January 22 analyst meeting, including site consolidations. There continues to be good progress on executing these plans, with, for example, a high rate of acceptance by Ann Arbor colleagues who have been offered the opportunity to relocate to other Pfizer R&D sites.
Pfizer also announced that, after a distinguished career in which he made important contributions, Chief Financial Officer Alan Levin has resigned to pursue career opportunities outside Pfizer.
Topics
Organizations
Other news from the department people

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Ashland to increase solvent and emulsion pressure-sensitive adhesives prices for North America

BASF’s first production plant for chemical catalysts in Asia opened
Successful start-up of Perstorp’s new major Oxo investment in Sweden
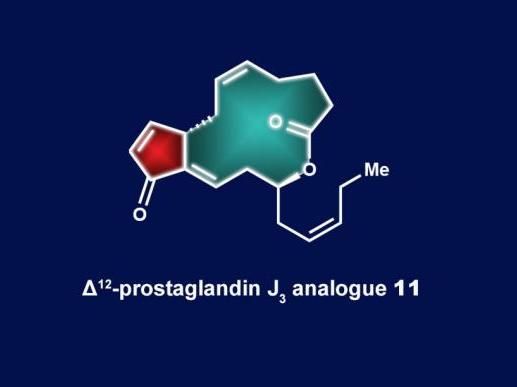
Rice University lab simplifies total synthesis of anti-cancer agent
Category:Chemical_companies_of_Canada
ENSR International Supports New Quality Standard for the Chemical Industry - Pilot Program at Arch Chemicals Earns Responsible Care(R) RC14001 Certification

LANXESS with strong third quarter of 2021 - Sales up 33.5 percent year-on-year at EUR 1.951 billion
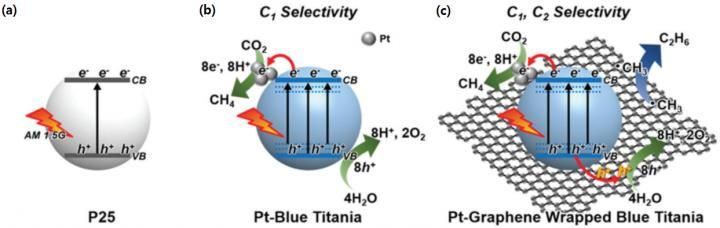
Converting carbon dioxide into methane or ethane selectively