New non-steroid cream Elidel® offers relief to babies suffering from eczema
The itching skin condition atopic eczema can be kept under control in 7 out of 10 babies with the new non-steroid cream Elidel®, one of the first new treatments to be developed for eczema in almost 50 years. Study results presented this week at the European Dermatology and Venereology Congress in Munich, showed that one week after starting treatment with Elidel, itching stopped or was only mild in 70% of 3- to 23-month-olds. By using the cream as soon as the first signs of eczema or itching recurred, disease flares were prevented in 70% of the babies and topical corticosteroid treatment was not required during the 6-month study. These results build on existing data already presented in patients aged 2-17.*
"Eczema in infants has a huge impact on the entire family", said one of the investigators taking part in the 250-patient study, Dr Mark Goodfield, Consultant Dermatologist at Leeds General Infirmary, UK. "For many patients, the itch is unbearable, the babies cannot sleep and their crying is upsetting for the whole family. When the infants are old enough to scratch themselves, they may scratch until they bleed and this, too, is particularly distressing for parents. So any relief for the baby brings relief for the whole family."
Eczema has typically been treated with moisturisers for the dry skin stage, and intermittent use of corticosteroids to treat disease flares in which the skin becomes increasingly red, swollen, and may weep and form crusts. However, corticosteroids have to be used with caution in infants or young children, and their long-term use can cause side-effects such as skin thinning and growth retardation.
"Many parents are reluctant to use corticosteroids on children, and so there is a great need for an effective alternative," Dr Goodfield said. "This study shows that Elidel cream has the potential to provide the sought-after alternative treatment which does not have steroid-associated side effects."
Novartis has filed applications with the Food and Drug Administration in the US, in Canada, and with health authorities in Switzerland and Denmark (the reference member state for the European Union), for authorisation to market Elidel.
Other data reported at the congress showed that:
· In this study with 250 infants, only 32% of those receiving a conventional treatment (emollients for dry skin and corticosteroids for flares) were flare-free over 6 months · In a year-long study involving 713 patients aged 2 – 17 years, 51% of the children using Elidel at the first signs and symptoms of eczema did not have any disease flares or need topical corticosteroids. Only 28% of patients receiving the conventional treatment were flare-free over the same period.
The most common adverse event reported among the 1700 patients treated with Elidel to date is a sensation of warmth or burning where the cream is applied. This is usually mild and disappears within a few days of treatment. It occurred in 1 of 10 children aged 2 – 17 years.
Elidel (pimecrolimus, formerly known as SDZ ASM 981), which is being developed by Novartis, is the first treatment proven to affect the long-term course of atopic eczema by reducing the incidence of eczema flares and the need for topical corticosteroids. As a skin-selective inflammatory cytokine inhibitor, Elidel works by selectively targeting those cells in the skin which release the pro-inflammatory mediators in atopic eczema.
Most read news
Topics
Organizations
Other news from the department research and development

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Pörner Group signs contract to construct a new Biturox-plant in Morocco
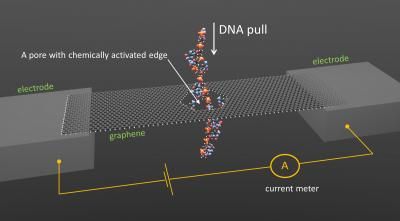
Simulation of fast, accurate DNA sequencing through graphene nanopore
A milestone for new carbon-dioxide capture/clean coal technology

Clariant divests its Pigments business - Closing anticipated in the first half of 2022
Expansion of Sequence Data Drives Biochemical Reagent Usage