Eisai Submits New Drug Application for Rheumatoid Arthritis Drug Adalimumab (D2E7)
Advertisement
Eisai Co., Ltd and Abbott Japan Co., Ltd submitted a new drug application (NDA) to the Minister of Health Labour and Welfare (MHLW) for the manufacturing and marketing for the rheumatoid arthritis drug adalimumab (D2E7), which is co-developed by the two companies in Japan.
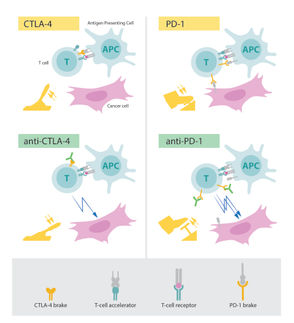
Adalimumab (D2E7) resembles antibodies normally found in the human body. It works by neutralizing TNF-alpha, a protein that plays a central role in the inflammatory responses of autoimmune disease such as rheumatoid arthritis.
Clinical trials for adalimumab (D2E7) have been conducted in Japan since 2000. The clinical trials have explored adalimumab's (D2E7) effectiveness in treating autoimmune disorders such as rheumatoid arthritis. Approximately 400 Japanese patients have participated in the trials.
Adalimumab (D2E7) is the only fully human monoclonal antibody approved by the United States Food and Drug Administration and the European Medicines Agency for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage and improving physical function in adult patients with moderately to severely active rheumatoid arthritis. Abbott markets adalimumab (D2E7) in the U.S. and European Union under the brand name of HUMIRA(R).
Most read news
Organizations
Other news from the department research and development

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.