Acambis completes delivery of 500,000 doses of MVA3000 smallpox vaccine to US Government
Acambis plc announced that it has completed the delivery under contract terms of 500,000 doses of its investigational MVA attenuated smallpox vaccine, MVA3000, to the National Institute of Allergy and infectious diseases ("NIAID"), part of the US National Institutes of Health. The delivery fulfils a core requirement of the contract that was awarded to Acambis by the NIAID in September 2004 for the manufacture and development of an MVA vaccine.
Acambis is co-developing MVA3000 with Baxter Healthcare SA ("Baxter"), which produced the 500,000 doses at its facilities and provides process development and manufacturing services.
In August, the Department of Health and Human Services issued a Request for Proposals (RFP) for the manufacture of up to 20 million doses of MVA attenuated smallpox vaccine and advanced clinical testing up to and including obtaining a product license for MVA. It also included options for the purchase of up to 60 million additional doses of MVA and "warm-base" manufacturing over the longer term. Acambis submitted a proposal in response to the RFP in October and the US Government has indicated that contract award(s) will be made in February 2006.
Organizations
Other news from the department research and development
These products might interest you

Good Weighing Practice by Mettler-Toledo
Your Concrete Weighing Quality Assurance Plan
GWP Verification service

Milli-Q® Services / MyMilli-Q™ by Merck
Services & Support for Water Purification Systems
Quality Care, Delivered. In Person & Online

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Labomatic and KNAUER enter into strategic cooperation - Customers in the field of liquid chromatography in particular are likely to benefit from the complementary expertise

Manufacturers in the laboratory sector choose analytica - Large number of exhibitors registered more than a year before analytica

Rousselet-Robatel - Annonay, France
Glutamate_carboxypeptidase_II
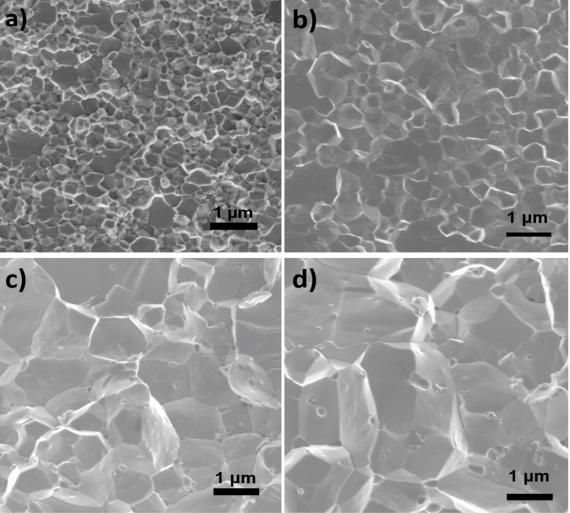
New thermoelectric material with high power factors

Twelve ideas for the defossilisation of the plastics industry - “Start-ups provide crucial impulses for the future of our industry”

Analytica 2016: Record number of exhibitors thanks to 6.5 percent increase - Several world premieres enthrall visitors
Construction to Begin at Emerald Kalama Chemical in the Netherlands for Non-Phthalate Plasticizers Manufacturing Operation
Rhodia to Close Carboxylation Unit at Leuna