Gel on Command
Switching between liquid and gel: counterion-dependent conformational change in molecular aggregates
Twisted nanostructures are an important biological motif - just think of the DNA double helix or proteins with helical sections important to their function. Researchers are anxious to produce artificial helices, which could be useful in nanotechnological applications. Korean researchers have now successfully created a molecular system that can even form helices "on demand", turning the initially liquid solution into a gel.
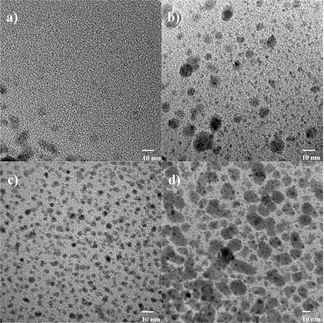
A team at Yonsei University in Seoul, Korea, headed by Myongsoo Lee, have developed a special type of molecule as the basic building block for their helices. This involves a base consisting of three aromatic rings which is bent like a boomerang. The central ring has a long, branched side-chain hanging from it. When a silver salt is added to a solution of these molecules, complexes form between the molecules and the positively charged silver ions; the "boomerangs" really get a hold on the silver ions. If the negatively charged counterion in the silver salt is boron tetrafluoride (BF4-), the complexes pile up into long, twisted columns. The BF4- ions fit exactly into the cavity that remains inside the "belly" of the helices and stabilize them. This results in a surprise: The liquid turns into a jelly-like mass. How does this happen? It turns out that the helices aggregate into regular bundles of fibers, which get tangled up with each other to form an interwoven, three-dimensional network. The liquid remains trapped inside this fibrous framework; this forms a gel, a kind of intermediate between a liquid and a solid. If a fluoride salt is then added to the gel, it liquefies. This is a result of the enormous attraction of the fluoride ions (F-) for the silver ions, which are lured out of their complexes. The fibrous aggregates collapse back into individual molecules. This effect is reversible if the fluoride ions are trapped by the addition of other salts.
If salts containing the C2F5CO2- ion are added to the gel, it also liquefies. Electron microscopy images show that in this case, the phenomenon has a different cause. The complexes do not fall apart into individual molecules, but form a different structure instead. Instead of interwoven helical columns, they form individual zigzagging bands. The reason for this change in structure is the difference in size of the anions: C2F5CO2- is bigger than BF4- and thus does not fit into the cavity inside the helices, which are thus not stabilized. The result of all this is the birth of a new type of "intelligent" nanomaterial whose properties can be switched solely by the choice of counterion.
Original publication: M. Lee; "Stimuli-Responsive Gels from Reversible Coordination Polymers"; Angewandte Chemie International Edition 2005, 44, 5810.
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.