Norvatis: New study shows Elidel® provides sustained control of eczema
Treatment with ElidelO (pimecrolimus) Cream 1% provided sustained control of the symptoms associated with atopic eczema for up to 18 months in both adult and paediatric patients, according to new study results announced during the congress of the American Academy of dermatology in New Orleans, USA.
The objective of the multinational study was to evaluate long-term efficacy and safety of Elidel in paediatric and adult patients with eczema of any severity, who had previously completed a six-month core study demonstrating the effectiveness of Elidel in controlling the acute signs and symptoms of atopic eczema. The extension study included 368 of these patients, who were treated for up to 18 months. Patients experienced sustained relief of their eczema symptoms (up to 18 months) when treated with Elidel in a naturalistic setting, i.e. in a clinical study that closely replicated real life. In the core study, 79.6% of patients who had used Elidel twice-daily experienced relief from the itching (pruritis) associated with mild to moderate eczema, defined as an improvement in symptoms to absent/mild pruritus.
Most read news
Other news from the department research and development

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

From scrap to raw material: With artificial intelligence to an efficient circular economy - Car2Car project develops technologies for an optimized recycling of end-of-life vehicles
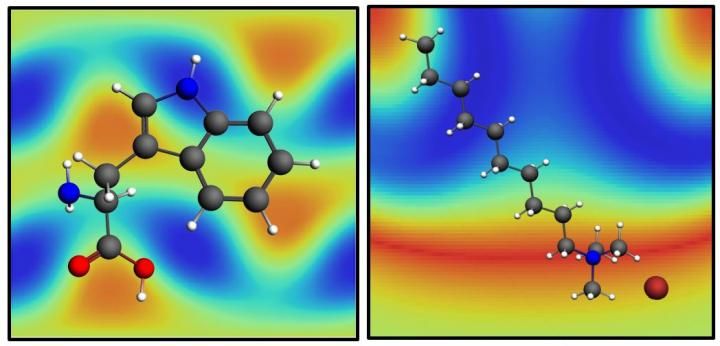
Good vibrations help reveal molecular details

Physicists propose a new method for monitoring nuclear waste - Scenarios for using neutrino detectors in nuclear interim storage facilities

Brenntag acquires North American acetone specialist Matrix Chemical
SABIC selects Basell's Hostalen technology for two new HDPE plants in Europe and Saudi Arabia
Affymetrix and Iconix Collaborate to Develop New Solutions for Assessing Drug Toxicity
Electrons zipping around in crystals

Lignovations Closes Seed Investment to Accelerate Lignin-Based Biomaterial Innovation - A multifunctional alternative to many synthetic chemicals in cosmetics, coatings, packaging, adhesives, and other applications
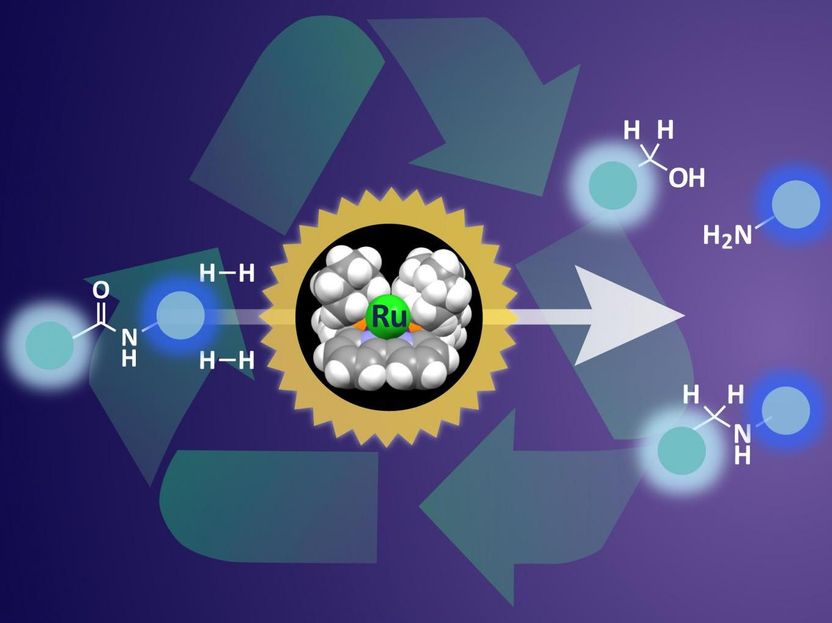
Breaking down plastic waste - Organo-ruthenium mediated hydrogenation under mild conditions
AkzoNobel to outline new strategy - Plan for enhanced value creation