Tolerability Demonstrated by ABX-EGF as Monotherapy in Advanced Cancers
Researchers presented preliminary results from a Phase 1 clinical trial of ABX-EGF, the only fully human monoclonal antibody in development against the epidermal growth factor receptor (EGFr), a receptor identified in many solid tumor types. Co-developed by Abgenix, Inc. (Nasdaq: ABGX) and Immunex Corporation (Nasdaq: IMNX), ABX-EGF was studied as monotherapy (without concomitant chemotherapy) in patients with various types of cancer. Data were presented today at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO).
Data from the ongoing, multiple-dose Phase 1 study of ABX-EGF included the results from 28 patients with various types of advanced solid refractory tumors, including kidney, prostate, pancreatic, non-small cell lung, colorectal and esophageal cancer. Patients received ABX-EGF by intravenous infusion every week for four weeks and were followed for safety for an additional five weeks. Doses ranged from 0.01 mg/kg to 1.0 mg/kg of ABX-EGF preceded by a loading dose. The primary objective of this Phase 1 study is to evaluate the safety of ABX-EGF at multiple dose levels.
"ABX-EGF’s role in targeting the EGF receptor and blocking the growth of tumor cells make it an exciting and promising research candidate in the treatment of cancer," said Robert Figlin, M.D., professor of Medicine and Urology of the Johnsson Comprehensive Cancer Center at the UCLA School of Medicine and Phase 1 lead investigator. " I am encouraged by plans to investigate its potential as a single-agent against multiple tumor types."
The following data were presented at the ASCO meeting:
Multiple doses of ABX-EGF appear to be well tolerated at doses ranging up to 0.75 mg/kg with a loading dose of 1.0 mg/kg. No antibody formation to the molecule was detected in any patient receiving ABX-EGF. No allergic reactions or infusion-related reactions were observed in any patient receiving ABX-EGF. Pharmacokinetic observations indicated that serum levels of ABX-EGF associated with efficacy in mouse xenograft models were attained in patients at a dose of 1.0 mg/kg. Skin rashes characteristic of EGF receptor targeting agents were observed at a dose of 1.0 mg/kg of ABX-EGF when preceded by a 2.0 mg/kg loading dose. Stable disease has been achieved in two patients who received low doses (0.1 and 0.75 mg/kg) of ABX-EGF as monotherapy.
Based on the preliminary results of the Phase 1 study, Abgenix and Immunex initiated a Phase 2 study of ABX-EGF in kidney cancer. The companies plan to initiate a series of Phase 2 clinical trials in additional cancer indications.
Topics
Organizations

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Bioswale
Golden_beryl
HLA-Cw*16
BASF temporarily idles steam cracker in Ludwigshafen - Order levels remain generally low
Actinometer
Tiger's_eye
Cytarabine
Detecting fake wine vintages: It's an (atomic) blast
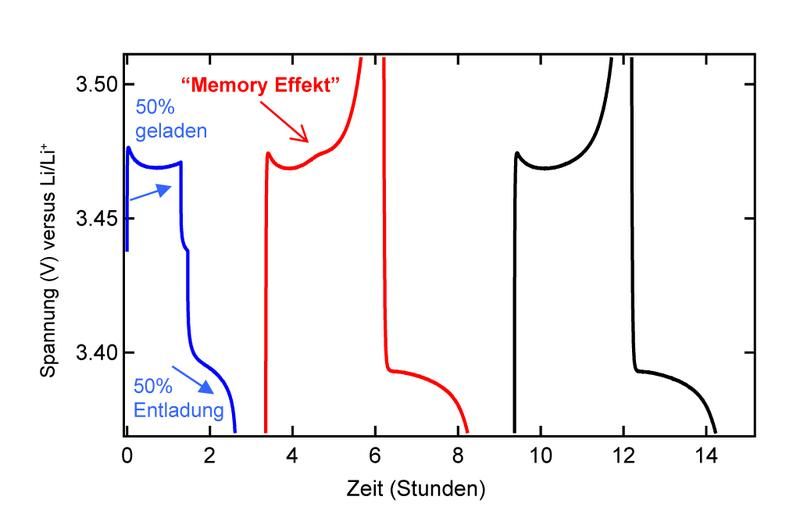
Memory effect now also found in lithium-ion batteries
Sigmatropic_reaction
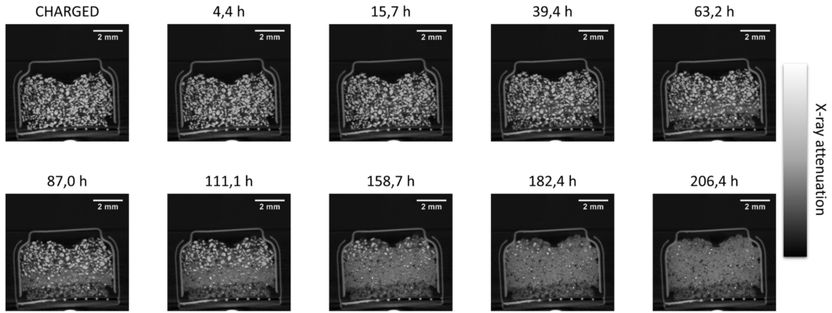
A fast 4D look into materials and substances - A project is developing an AI-based software framework that can process terabyte-sized 4D tomography data on a desktop PC