Dye Nanorods
Highly ordered dye aggregates through stepwise organization
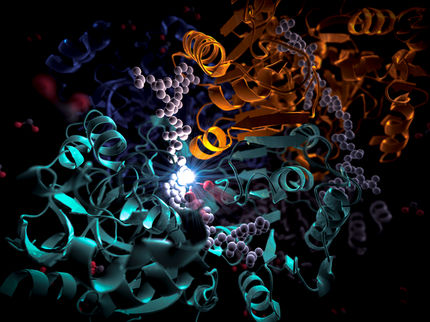
Plants and a whole series of bacteria can trap energy from sunlight. To harvest the light as effectively as possible and to convert it into chemical energy these species have highly developed photosynthesis systems. Hundreds of dye molecules aggregate together in highly ordered nanostructures. Naturally, scientists would love to be able to construct copies of such systems and thus generate novel materials for future opto-electronic components and effective solar cells. However, such highly organized architectures of dye molecules are difficult to create.
Frank Würthner and Sheng Yao from the University of Würzburg and Uwe Beginn from the RWTH Aachen have made a breakthrough. The researchers selected a so-called bis-merocyanin dye as the starting material for their structure. The dye molecule is made up of a central aromatic carbon ring which has three hydrocarbon "tails" and two merocyanin "arms". As the concentration of the dye increases a stepwise self-organization occurs. First, the arms of different molecules pair-up so that a long, disordered, polymer chain is formed. These chains then organize themselves into helical strands. Then, six of these helices twist around each other to form long cylinders. In this structure all the hydrocarbon tails point outwards. If the concentration is increased further the tails force their way into the neighboring cylinders, which organizes the cylinders into tight bundles with hexagonal packing. The result is a gel with liquid-crystal characteristics.
"The construction by self organization and the cylinder-like structure of this surprisingly well-defined dye aggregate is reminiscent of the natural chlorophyll rod aggregates in photosynthetic bacteria. However, the performance of the artificial dyes is still not comparable with that of their natural counterparts," explains Würthner. "By means of structural modifications we hope to be able to make the step to functional complexity in the next few years."
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.