Guanidinium stabilizes perovskite solar cells at 19 percent efficiency
With the power-conversion efficiency of silicon solar cells plateauing around 25%, perovskites are now ideally placed to become the market's next generation of photovoltaics. In particular, organic-inorganic lead halide perovskites offer manufacturing versatility that can potentially translate into much higher efficiency: studies have already shown photovoltaic performances above 20% across different solar cell architectures built with simple and low-cost processes.
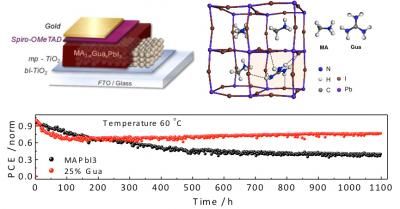
Stability test of the novel MA(1-x)GuaxPbI3 perovskite material under continuous light illumination compared with the state-of-the-art MAPbI3. A schematic of the device architecture and the simulated crystalline structure is also provided.
M.K. Nazeeruddin/EPFL
The main challenge for the perovskite field is not so much efficiency but stability. Unlike silicon cells, perovskites are soft crystalline materials and prone to problems due to decomposition over time. In a commercial context, this puts perovskites on a higher price tag than conventional silicon cells.
There have therefore been many efforts in synthesizing perovskite materials that can maintain high efficiency over time. This is done by introducing different cations (positively charged ions) into the crystal structure of the perovskite. Although success has been reported by mixing inorganic cations like cesium or rubidium into the perovskite composition, these solutions tend to be difficult and expensive to implement.
Meanwhile, no organic - and easier to synthesize - cations that can improve both efficiency and stability have been found so far. Now, the lab of Mohammad Khaja Nazeeruddin at EPFL Valais Wallis, with colleagues at the University of Cordoba, has discovered that they can improve perovskite stability by introducing the large organic cation guanidinium (CH6N3+) into methylammonium lead iodide perovskites, which are among the most promising alternatives in the group today.
The scientists show that the guanidinium cation inserts into the crystal structure of the perovskite and enhances the material's overall thermal and environmental stability, overcoming what is known in the field as the "Goldschmidt tolerance factor limit." This is an indicator of the stability of a perovskite crystal, which describes how compatible a particular ion is to it. An ideal Goldschmidt tolerance factor should be below or equal to 1; guanidinium's is just 1.03.
The study found that the addition of guanidinium significantly improved the material stability of the perovskite while delivering an average power conversion efficiency over 19% (19.2 ± 0.4%) and stabilizing this performance for 1000 hours under continuous light illumination, which is a standard laboratory test for measuring the efficiency of photovoltaic materials. The scientists estimate that this corresponds to 1333 days (or 3.7 years) of real-world usage - this is based on standard criteria used in the field.
Professor Nazeeruddin explains: "Taking a standard acceleration factor of 2 for each ten degrees increase in temperature, an acceleration factor of 8 is estimated for 55 °C as opposed to 25 °C degrees. Hence the 1000 hours at 55°C equivalent would be 8000 hours. Our cells were subjected at 60°C, therefore the numbers could be even higher. Assuming the equivalent of 6 hours full sunlight/day, or 250Wm-2 average irradiance (equivalent to North Africa) the total number of days are 1333, equals to 44.4 months and 3.7 years stability. However, for the standard solar cell accreditation a series of stress tests including temperature cycling and damp heat are also required."
"This is a fundamental step within the perovskite field," says Nazeeruddin. "It offers a new paradigm in perovskite design as further explorations beyond the tolerance factor limit could prevail for cationic blends while preserving a 3D structure with improved stability through increased number of H-bonds within the inorganic framework - a problem that we are now close to solving."
Original publication
Alexander D. Jodlowski, Cristina Roldán-Carmona, Giulia Grancini, Manuel Salado, Maryline Ralaiarisoa, Shahzada Ahmad, Norbert Koch, Luis Camacho, Gustavo de Miguel, Mohammad Khaja Nazeeruddin; "Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells"; Nature Energy; 2017