Catalyzing carbon dioxide
On any given day, more than 2 million pounds of carbon dioxide are pumped into the atmosphere from factories, emissions from cars and trucks and the burning of coal and natural gas to generate electricity.
For many, it's a cause for environmental concern, but for Haotian Wang, it's the perfect raw material.
A Fellow at the Rowland Institute at Harvard, Wang and his research team have developed a system that uses renewable electricity to electrochemically transform carbon dioxide into carbon monoxide - a key commodity used in any number of industrial processes. The energy conversion efficiency from sunlight to CO can be as high as 12.7 %, more than one order of magnitude higher than natural photosynthesis.
"Basically, what this is is a form of artificial photosynthesis," Wang said. "In a plant, sunlight, CO2 and water become sugar and oxygen. In our system, the input is sunlight, CO2 and water, and we produce CO and oxygen."
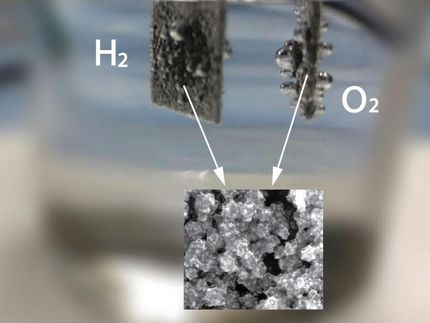
That reaction takes place in an unassuming-looking device, barely the size of a smartphone, that includes two electrolyte-filled chambers separated by an ion exchange membrane.
On one site, an electrode powered by renewable energy oxidizes water molecules into oxygen gas and frees protons. These protons move to the other chamber where - with the help of a carefully designed metal single atom catalyst - they bind to carbon dioxide molecules, creating water and carbon monoxide.
"The challenge is that most catalysts that are known tend to produce hydrogen gas," Wang said. "So it's difficult, when you split water, to prevent those protons from combining together to form hydrogen gas. What we needed was a catalyst that can prevent hydrogen evolution and instead can efficiently inject those protons into CO2, therefore achieving a high selectivity for CO2 reduction."
Unfortunately, the two best-known such catalysts are gold and silver - precious metals that are very costly to make the reaction cost effective on a large scale.
"So we began by looking at low-cost materials like nickel, iron and cobalt, which are all Earth-abundant," Kun Jiang said, who is a postdoctoral fellow in Wang group and the first author of this work. "But the problem is that they are all very good hydrogen catalysts, so they want to produce hydrogen gas.
In addition, they can all very easily be poisoned by carbon monoxide," he added. "Even if you manage to use them to reduce CO2, the resulting CO bonds very strongly to the surface, preventing any further reactions from taking place."
To solve those problems, Wang and his Stanford collaborators, Prof. Yi Cui and Prof. Jens Nørskov, set about working to "tune" the electronic properties of the metals. Dr. Samira Siahrostami, a staff scientist from Prof. Nørskov group rationalized the nature of active sites by atomic scale modeling and discovered that dispersing nickel metals into isolated single atoms, which are trapped in graphene vacancies, produced a material that was eager to react with carbon dioxide and willing to release the resulting carbon monoxide.
That carbon monoxide, Wang said, can then be used in a host of industrial processes.
"Carbon monoxide is a very important industry product," Wang said. "It can be used in plastics production, to make hydrocarbon products or can be burned as a fuel itself. It's widely used in industry."
Ultimately, though, the hope is that the system could one day be scaled up enough to scrub carbon dioxide from the atmosphere in an effort to combat global climate change.
"The basic idea was if we can capture existing CO2 and use renewable electricity, from solar or wind power, to reduce it into useful chemicals," Wang said, "then we can possibly form a carbon loop."
Original publication
Kun Jiang, Samira Siahrostami, Austin J. Akey, Yanbin Li, Zhiyi Lu, Judith Lattimer, Yongfeng Hu, Chris Stokes, Mahesh Gangishetty, Guangxu Chen, Yawei Zhou, Winfield Hill, Wen-Bin Cai, David Bell, Karen Chan, Jens K. Nørskov, Yi Cui, Haotian Wang; "Transition-Metal Single Atoms in a Graphene Shell as Active Centers for Highly Efficient Artificial Photosynthesis"; Chem; 2017
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.