Flipping the electron spin
Test procedure for developing quick-charging lithium-ion batteries
Advertisement
When lithium-ion batteries are charged too quickly, metallic lithium gets deposited on the anodes. This reduces battery capacity and lifespan and can even destroy the batteries. Scientists at the Forschungszentrum Jülich and the Technical University of Munich (TUM) have now presented a process that, for the first time ever, allows this so-called lithium plating process to be investigated directly. This puts new strategies for quick-charging strategies close at hand.

Test cell for lithium ion batteries
Forschungszentrum Jülich / T. Schlößer
Technische Universität München, J. Wandt / Forschungszentrum Jülich, J. Granwehr

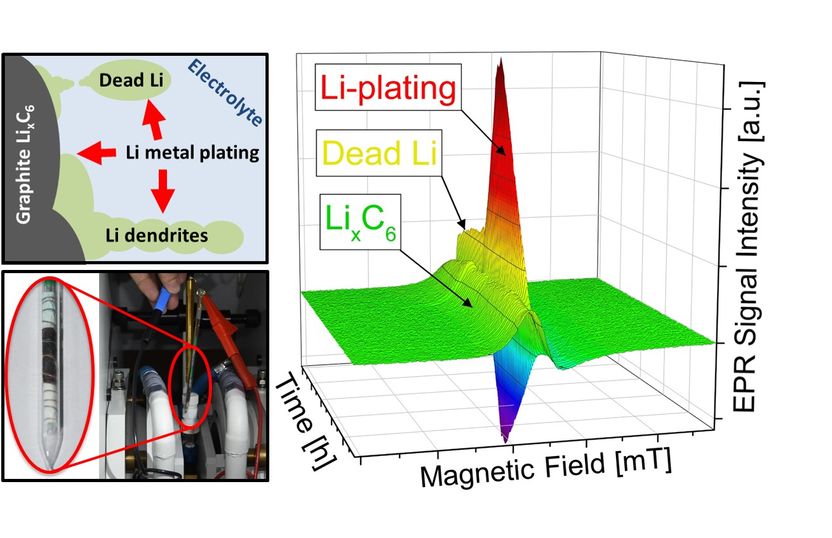
Lithium plating, the depositing of metallic lithium at the anodes of lithium-ion batteries, is one of the primary factors that limits charging current. The performance of batteries suffers significantly from these metallic deposits. In extreme cases this can result in short circuits and even batteries going up in flames.
When charging batteries, the positively charged lithium ions move through the liquid electrolytes and are deposited in the porous graphite anodes. However, the larger the current and the lower the temperature, the greater the probability that the lithium ions will not be deposited within the electrodes, as desired, but rather as a solid metallic layer on the outer surface.
Indirect evidence does not serve the goal
Even though this phenomenon is basically well-known, many aspects remain shrouded in mystery. Until now, it was not possible to directly observe how and under which circumstances lithium plating takes place. "Using traditional methods of microscopy, we can only observe a battery after the fact, because it needs to be cut open," explains Dr. Josef Granwehr at the Jülich Institute of Energy and Climate Research (IEK-9). "In the process, further reactions that distort the results become inevitable."
Even highly developed processes like neutron scattering allow for only indirect analyses. Compounding the problem is the fact that available slots for measurements at research reactors and large particle accelerators are scarce. This makes these tools more suitable for fundamental investigations than for tedious, practical test series.
Electrons show the way
The electron paramagnetic resonance (EPR) spectroscopy process presented in the renowned scientific journal Materials Today, on the other hand, can be readily integrated into laboratory procedures – with only moderate investment. The method is akin to the better-known nuclear magnetic resonance (NMR) spectroscopy, albeit focusses on electron spins rather than atomic nuclei.
"Electrons are placed in an externally applied, static magnetic field," explains Granwehr. Unpaired electrons in the sample are "sounded out" using microwaves. In the magnetic field, these stimulate the electrons to flip, which can be measured via the associated drop in microwave radiation intensity. EPR can differentiate between metallic lithium plating and lithium embedded in the graphite anodes.
The test cell is the key
"The key to detecting lithium plating using EPR was the construction of a test cell compatible with the requirements of EPR spectroscopy while at the same time exhibiting good electrochemical properties," explains lead author Dr. Johannes Wandt. "The geometry is also important. Precise measurement results are contingent on the sample being exposed to the magnetic field but not the inevitably present electric field."
To ensure this, Wandt developed a rod-shaped cell while he was a doctoral candidate in the group of Prof. Hubert A. Gasteiger (Chair of Technical Electrochemistry) at TUM that allows the formation of metallic lithium to be detected directly and with quantitative precision.
The right strategy for quick charging
"Using this process, it is now for the first time possible to investigate lithium plating and the associated processes in a differentiated manner that is relevant to a whole array of applications," explains Rüdiger-A. Eichel, a director at the Jülich Institute of Energy and Climate Research (IEK-9). "One example is the development of safe and at the same time fast charging protocols. Our process make determining the maximum charging current before lithium plating sets in possible, as well as ascertaining other boundary conditions like temperature and the influence of electrode geometry."
Beyond this, the methodology is well suited as a test procedure for a variety of battery materials, for example the development of new admixtures that suppress lithium plating.