Sulfur improves birefringence!
Developing liquid crystalline molecules
Advertisement
A team of researchers led by Assistant Professor Yuki Arakawa, Toyohashi University of Technology, has successfully liquid crystallized π-conjugated 2 rod-like molecules with alkylthio groups containing sulfur 1 , and developed high birefringence molecules that exhibit nematic liquid crystal with high fluidity in temperature ranges including room temperature. This molecular design is expected to offer a new liquid crystal material that contributes to the high quality image resolution of liquid crystal displays.
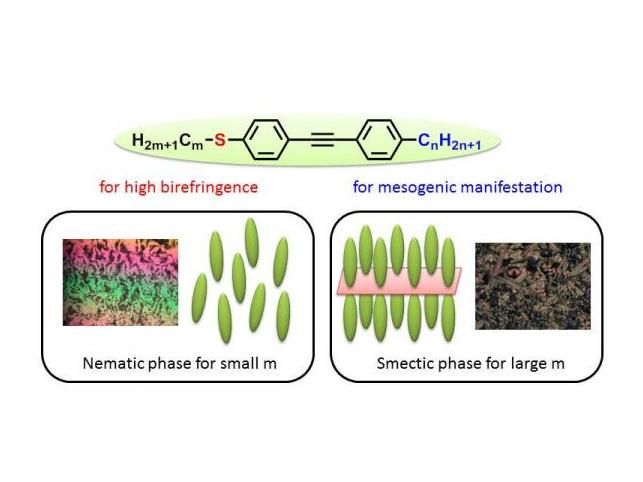
Images of alkylthio group rod-like molecules exhibiting liquid crystallinity at room temperature, and the phase structures.
COPYRIGHT (C) TOYOHASHI UNIVERSITY OF TECHNOLOGY. ALL RIGHTS RESERVED.
Liquid crystal materials with high birefringence and dielectric constant have been contributing to lowering the driving-voltage and improving the response speed of liquid crystal (LC) displays. Recently, various approaches have been taken to apply high birefringence LC materials to broadband circularly polarized light-reflecting films for brightness enhancement film, or to cholestric LC lasers for continuous oscillation.
In terms of practicality, LC materials need to be developed by either forming LC phases in room temperature or fixing the orientation state of LC. However, improving birefringence and dielectric constant requires both an anisotropic molecular structure and electron richness, making a rise in the phase transition temperature (especially melting point) inevitable due to large intermolecular forces. In short, it is difficult to form a liquid crystalline state under room temperature.
Assistant Professor Yuki Arakawa and his team took interest in alkylthio groups (SCmH2m+1) that contain "sulfur", a component of hot springs and one of the few surplus resources Japan has. Although alkylthio groups have high polarizability and are expected to be an effective substitutional group for birefringence improvement, only a few successful cases of rod-like molecules with alkylthio groups forming liquid crystals have been reported due to their difficulty to crystalize.
Assistant Professor Yuki Arakawa and his team introduced substantially long alkyl chains having five or more carbons to one terminal of a diphenylacetylene structure 3 with alkylthio groups to reveal that liquid crystallinity is exhibited during the cooling process. This is considered to be due to the fact that among the molecules aligned antiparallelly, long alkyl groups inhibit molecular crystalline packing and thus enable the molecules to rotate and translate while maintaining their orientation, which eventually leads to the formation of a liquid crystal phase. Furthermore, the team observed a phenomenon where the melting point decreased due to the large bending and low electron donating properties of the alkylthio groups, and succeeded in developing a molecule that exhibits liquid crystallinity in temperature ranges including room temperature. Changing the carbon numbers in alkylthio groups after introducing long alkyl chains enables the formation of both a highly-ordered smectic phase with a high viscosity layer structure and a nematic (N) phase with low viscosity, which is particularly important for optical applications. Comparison with oxygen analogues 4 confirmed significant improvement of optical properties.
"There were only a few reports on rod-like structure molecules with alkylthio groups exhibiting liquid crystalline phases, and no studies revealed the characteristics of these molecules, including the reason why they tend not to form liquid crystalline phases. We're now aiming to utilize the characteristics of each phase to the full to explore various optical and electronic physical properties, including not only optical properties but also semiconductor properties," says Assistant Professor Arakawa.
































































