'Ion billiards' cue novel material synthesis method
A team of Hokkaido University researchers has developed a novel material synthesis method called proton-driven ion introduction (PDII) which utilizes a phenomenon similar to "ion billiards." The new method could pave the way for creating numerous new materials, thus drastically advancing materials sciences.
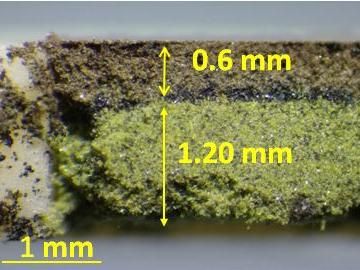
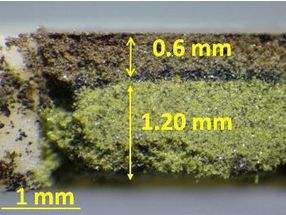
Na+ of Na3V2(PO4)3 was substituted with K+, producing a thermodynamically metastable material which cannot be obtained using the conventional solid-state reaction method.
Fujioka M. et al., Journal of the American Chemical Society, November 16, 2017
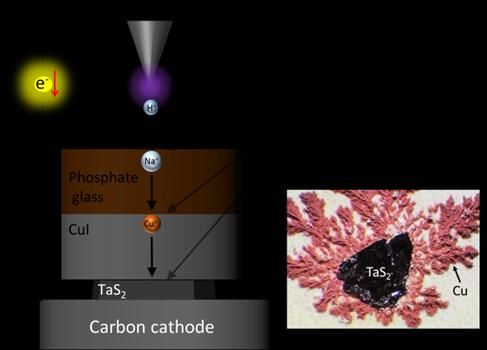
Hydrogen ions (H+) force out the Sodium ions (Na+) from Phosphate glass, and then the Sodium ions (Na+) force out Cupper ions (Cu+) from CuI, shooting the Cu+ into nanometer-level gaps in TaS2. Excessive Cu+ formed Cupper metals which crystalized around TaS2 (right image).
Fujioka M. et al., Journal of the American Chemical Society, November 16, 2017
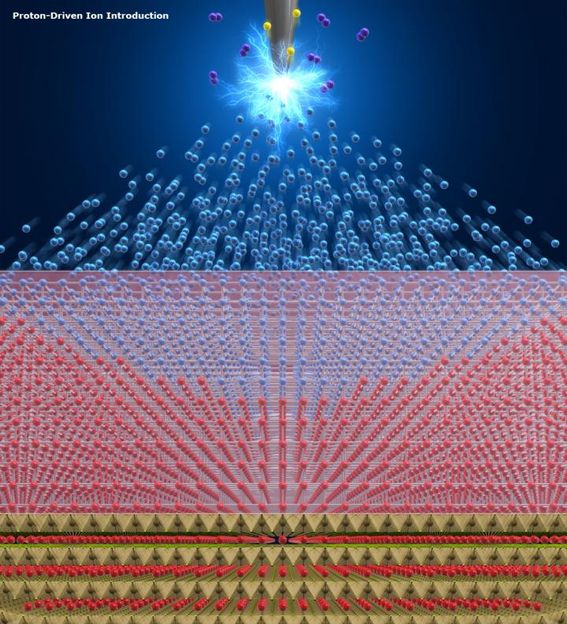
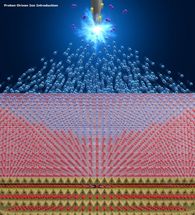
Protons generated by electric disassociation of hydrogen are shot into the supply source of the desired ions. The ions are then forced out of the source to be introduced into the host material.
Fujioka M. et al., Journal of the American Chemical Society, November 16, 2017
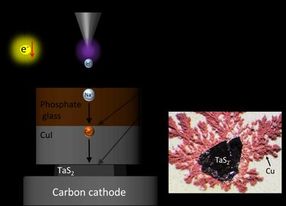
The synthesis method is based on a liquid-free process that allows for intercalation - insertion of guest ions into a host material - and ion substitution with those in the host material by driving ions with protons. This study, was led by Assistant Professor Masaya Fujioka and Professor Junji Nishii at the university's Research Institute for Electric Science.
Conventionally, intercalation and ion substitution have been conducted in an ion solution, but the process is regarded as cumbersome and problematic. In a liquid-based process, solvent molecules can be inserted into the host materials along with guest ions, degrading the crystal quality. It is also difficult to homogeneously introduce ions into host materials, and some host materials are not suitable when used with liquids.
In the PDII method, a high voltage of several kilovolts is applied to a needle-shaped anode placed in atmospheric hydrogen to generate protons via the electrolytic disassociation of hydrogen. The protons migrate along the electric field and are shot into the supply source of the desired ions - similar to balls in billiards - and the ions are driven out of the source to keep it electrically neutral. Ions forced out of the source are introduced, or intercalated, into a nanometer-level gap in the host material.
In this study, by using different materials as ion supply sources, the team succeeded in homogenously introducing lithium ions (Li+), sodium ions (Na+), potassium ions (K+), copper ions (Cu+) and silver ions (Ag+) into nanometer-level gaps in tantalum (IV) sulfide (TaS2), a layered material, while maintaining its crystallinity. Furthermore, the team successfully substituted Na+ of Na3V2(PO4)3 with K+ , producing a thermodynamically metastable material, which cannot be obtained using the conventional solid-state reaction method.
"At present, we have shown that hydrogen ions (H+), Li+, Na+, K+, Cu+ and Ag+ can be used to introduce ions in our method, and we expect a larger variety of ions will be usable. By combining them with various host materials, our method could enable the production of numerous new materials," says Masaya Fujioka. "In particular, if a method to introduce negatively charged ions and multivalent ions is established, it will spur the development of new functional materials in the solid ion battery and electronics fields."
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.



























































