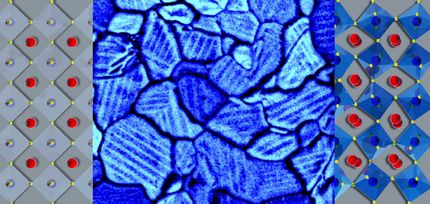
A new spin to engineer better absorptive materials
A team of University of Illinois bioengineers has taken a new look at an old tool to help characterize a class of materials called metal organic frameworks - MOFs for short. MOFs are used to detect, purify and store gases, and could help solve some of the world's most challenging energy, environmental and pharmaceutical challenges - they can even pull water molecules straight from the air to provide relief from drought.
University of Illinois bioengineers, from left, Ayanjeet Ghosh, professor Rohit Bhargava, Prabuddha Mukherjee and Sanghamitra Deb are using an updated infrared imaging technique to better examine and optimize a group of materials that could help solve some of the world's most challenging energy, environmental and pharmaceutical challenges.
Photo by L. Brian Stauffer
The research team, led by bioengineering professor Rohit Bhargava, is using infrared chemical imaging to examine and optimize the structure of MOFs. Although around for more than a decade, IR imaging is greatly underutilized in materials analysis. The researchers found that with a few modifications to improve the speed of analysis, it is the perfect tool for this application.
MOFs are microscopic-scale porous crystals engineered from metal ions bound together by organic molecules called ligands. Although they are tiny, they have an immense absorptive ability.
"The pores allow the MOFs to work like tiny sponges that can soak up chemicals such as pharmaceuticals and gases," said Sanghamitra Deb, a postdoctoral researcher at the Beckman Institute for Advanced Science and Technology at the U. of I.
"The precise structure and chemistry of MOFs greatly influence their functionality," said Prabuddha Mukherjee, a Beckman Institute research scientist. "Therefore, detailed characterization is essential in determining their best use."
The traditional methods used in materials science analysis, like high-powered electron microscopy and spectroscopy, do not combine chemical insights with the spatial resolution of IR imaging, the researchers said, so they can only provide average chemical measurements.
MOFs form by crystallizing out of a solution, and there is no way of fully controlling their structure or chemistry. "This lack of control leaves a lot of room for defects to form, and the traditional methods for characterization only tell us that there is a defect but cannot pinpoint the specific location," Mukherjee said.
"IR imaging allows us to see the chemistry and the structure in one shot," said Ayanjeet Ghosh, a postdoctoral researcher with the Beckman Institute. "We can resolve structures down to a few microns and determine their chemical composition over a few micron areas, understand how and why the spectra change as a function of space, and do it with a single analysis."
IR imaging also offers a unique scale range to work in, the researchers said.
"We do not need to see down to the atomic scale, like many high-powered electron microscopy methods offer," Deb said. "At that scale, it would take a very long time to scan devices made with MOFs, which are typically about a millimeter squared in size."
Finally, many of the other traditional techniques are destructive, meaning that once analyzed with one method, the sample cannot be examined with any additional tools. "We may be able to spot an aberration in chemistry via spectroscopy, but we don't have the opportunity to see where the defect actually exists using another method because the sample is now gone," Ghosh said. "With IR imaging, we can do both at the same time."
"This unique use of an older technique, but with new instrumentation, allows us to quickly determine the quality and best application for specific MOFs in a nondestructive way - something no other group has been able to do," Mukherjee said.
The group envisions this technique being used with other devices made under similar conditions, as well as uses outside of the materials science realm.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!