Biocatalysis gives access to novel substance classes and elusive species
Novel Manich catalysts and bicyclic carboxylic acid esters synthesised
The application of Enzymicals recombinant pig liver isoenzymes (rPLE) enables the synthesis of novel, optical pure diastereomers of substituted proline, which shows activity as anti-Mannich catalyst. Additionally, rPLE is the key for successful production of a previously unavailable bicyclic carboxylic acid ester with four stereocenters as an interesting intermediate for pharmaceutical industry.

Fotolia
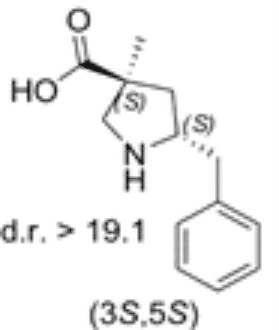
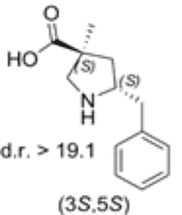
5-benzyl substituted (3S,5S)-Cα-methyl-β-proline
EnzymicalS

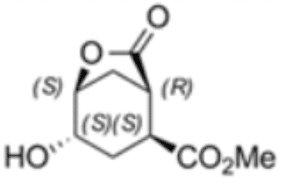
(1R,2S,4S,5S)-4-hydroxy-7-oxo-6-oxabicyclo [3.2.1] octane-2-carboxylic acid methyl ester
EnzymicalS
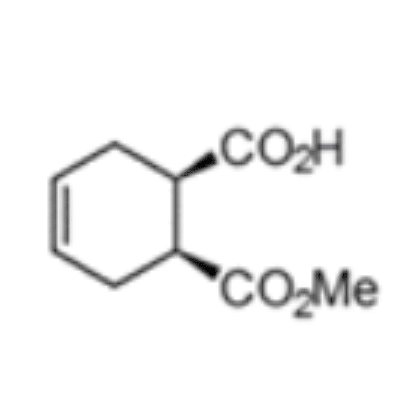
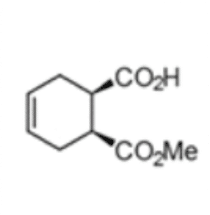
(1S,2R)-1-(methoxycarbonyl) cyclohex-4-ene-2-carboxylic acid
EnzymicalS

The asymmetric Mannich reaction is an important carbon-carbon bond forming reaction for the synthesis of β-amino carbonyl compounds. Consequently, the demand for Mannich reactions that selectively affords anti- or syn-products with high enantioselectivity is high. Beside proline which gives easy access to syn-products pyrrolidine derivatives catalyse enantioselective anti-Mannich reactions. But these are considerably rare.
The group of Professor Masterson (University of Southern Mississippi) has developed a new synthetic route to provide two diastereomers of 5-benzyl substituted Cα-methyl-β-proline in their optically pure form from malonate diester. For this rPLE isoenzymes from Enzymicals portfolio for the enzymatic hydrolysis and a new stereoselective cyclization strategy were utilized.
Both diastereomers of the 5-benzyl-Cα-methyl-β-proline analogue were tested for catalytic activity in the Mannich reaction between α-imino ester and isovaleraldehyde. The 5-benzyl substituted (3S,5S)-Cα-methyl-β-proline (1) proved to be an efficient anti-Mannich catalyst providing anti-products.
Having the proof-of-concept in hand, there are further options for optimization. Either for the chemo-enzymatic synthesis of the organocatalyst and the final anti-Mannich reaction.
The second example shows the utilization of a scaled-up biocatalytic method developed by Enzymicals as basis for the production of a previously unavailable intermediate with chemical name (1R,2S,4S,5S)-4-hydroxy-7-oxo-6-oxabicyclo [3.2.1] octane-2-carboxylic acid methyl ester (2). The four stereo centers of this bicyclic compound are a challenge for classical chemical synthesis. The synthesis of this stereo pure methyl ester was enabled by a combination of chemical and biocatalytic steps.
The chemoenzymatic process was originally developed for the production of (1S,2R)-1-(methoxycarbonyl) cyclohex-4-ene-2-carboxylic acid (3) by rPLE in an optimized enantioselective sequential multistep one pot reaction. Herein an optimized esterification protocol, starting from the economically favored meso-anhydride, was established and combined with the highly selective ECS-PLE06-catalyzed desymmetrization reaction. [2] The enantiopure compound (3) serves as starting material for the following oxidation and rearrangement reaction leading to ring formation. After an optimization of the process and the downstream procedure we are able to produce the new enantiopure intermediate in multi-hundred-gram scale.
The combination of biocatalysis with classical chemical synthesis and other stereo-selective techniques can bring a competitive advantage also for your research, development and production.
Original publication
Kotapati H.K., Robinson J., Lawrence D., Fortner K., Stanford C., Powell D., Wardenga R., Bornscheuer U.; "Diastereoselective hydrolysis of branched malonate diesters by Porcine Liver Esterase: Synthesis of 5-benzyl substituted Cα-methyl-β-proline and catalytic evaluation"; Eur JOC; 2017
Süss P., Borchert S., Hinze J., Illner S., v.Langermann J., Kragl U.m Bornscheuer U.T., Wardenga R.; "Chemoenzymatic Sequential Multistep One-Pot Reaction for the Synthesis of (1S,2R)-1-(Methoxycarbonyl)cyclohex-4-ene-2-carboxylic Acid with Recombinant Pig Liver Esterase"; Org. Process Res. Dev.; 2015
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.