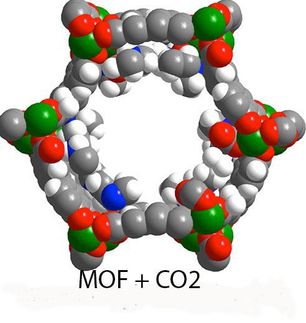
Taming defects in nanoporous materials to put them to a good use
Fundamental aspects of defective materials that can be employed to capture CO2
The word "defect" universally evokes some negative, undesirable feature, but researchers at the Energy Safety Research Institute (ESRI) at Swansea University have a different opinion: in the realm of nanoporous materials, defects can be put to a good use, if one knows how to tame them.
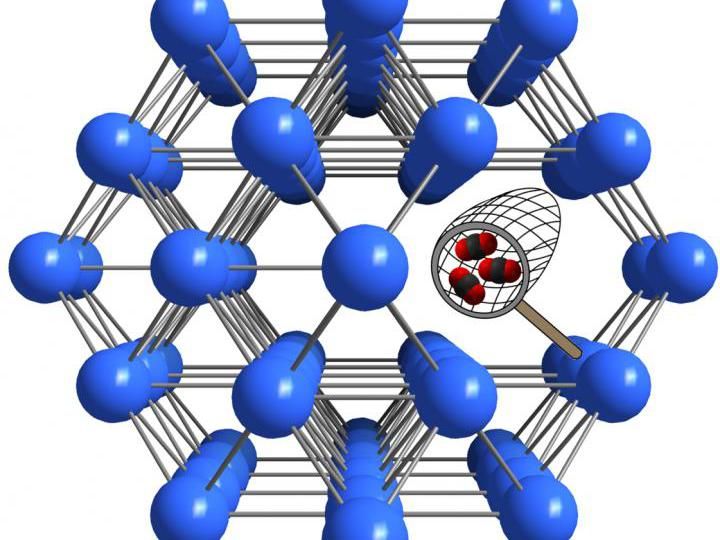
Modification of defective nanoporous materials has unique effects on their properties. Swansea University scientists are seeking to master this method to make new materials to capture CO2.
Swansea University
Metal Organic Frameworks
A team led by Dr Marco Taddei, Marie Sklodowska-Curie Actions Fellow at Swansea University, is investigating how the properties of metal-organic frameworks, a class of materials resembling microscopic sponges, can be adjusted by taking advantage of their defects to make them better at capturing CO2.
Dr Taddei said: "Metal-organic frameworks, or MOFs, are extremely interesting materials because they are full of empty space that can be used to trap and contain gases. In addition, their structure can be manipulated at the atomic level to make them selective to certain gases, in our case CO2."
"MOFs containing the element zirconium are special, in the sense that they can withstand the loss of many linkages without collapsing. We see these defects as an attractive opportunity to play with the properties of the material."
The researchers went on to investigate how defects take part in a process known as "post-synthetic exchange", a two-step procedure whereby a MOF is initially synthesized and then modified through exchange of some components of its structure. They studied the phenomenon in real time using nuclear magnetic resonance, a common characterization technique in chemistry. This allowed them to understand the role of defects during the process.
"We found that defects are very reactive sites within the structure of the MOF, and that their modification affects the property of the material in a unique way." said Dr Taddei "The fact that we did this by making extensive use of a technique that is easily accessible to any chemist around the globe is in my opinion one of the highlights of this work."
ESRI Research
ESRI Director, Professor Andrew Barron is co-author of the work, said: "In ESRI, our research efforts are focused on making an impact on the way we produce energy, making it clean, safe and affordable. However, we are well aware that progress in applied research is only possible through a deep understanding of fundamentals. This work goes exactly in that direction."
The study is a proof of concept, but these findings lay the foundation for future work, funded by the Engineering and Physical Sciences Research Council. The researchers want to learn how to chemically manipulate defective structures to develop new materials with enhanced performance for CO2 capture from steelworks waste gases, in collaboration with Tata Steel and University College Cork.
"Reducing the CO2 emissions derived from energy production and industrial processes is imperative to prevent serious consequences on climate," states co-author Dr Enrico Andreoli, Senior Lecturer at Swansea University and leader of the CO2 capture and utilization group within ESRI, "Efforts in our group target the development of both new materials to efficiently capture CO2 and convenient processes to convert this CO2 into valuable products."
Original publication
Original publication
Dr. Marco Taddei, Dr. Russell J. Wakeham, Athanasios Koutsianos, Dr. Enrico Andreoli ,Prof. Andrew R. Barron; "Post‐Synthetic Ligand Exchange in Zirconium‐Based Metal–Organic Frameworks: Beware of The Defects!"; Angew. Chem. Int. Ed.; 2018
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Archard_equation
A breakthrough in all-solid-state battery technology, enhancing the performance of the lithium from the bottom