Chemical hotspots
Scanning tunneling microscopy measurements identify active sites on catalysts
Chemistry live: Using a scanning tunneling microscope, researchers at the Technical University of Munich (TUM) were able for the very first time to witness in detail the activity of catalysts during an electro-chemical reaction. The measurements show how the surface structure of the catalysts influences their activity. The new analysis method can now be used to improve catalysts for the electrochemical industry.
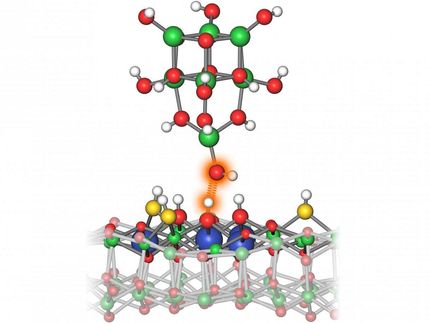
The analysis of the tunneling currents of a scanning tunneling microscope reveal the active sites on the catalyst surface.
Christoph Hohmann / NIM

Jonas Pfisterer and Yunchang Liang at the scanning tunneling microscope in the laboratory of Prof. Bandarenka, Physics of Energy Conversion and Storage at the Technical University of Munich.
Wenzel Schürmann / TUM

No energy transition without catalysts: On their own, the chemical processes necessary in order to manufacture hydrogen gas using electricity, to convert the hydrogen back into electrical energy in fuel cells, or to convert carbon dioxide into fuel are much too slow to be of practical use. Catalysts accelerate the reaction without being used up themselves.
"Catalysts are of enormous importance for the industry. Hence, the industry has a great interest in further improving the materials in order to increase the efficiency of the processes", explains Aliaksandr Bandarenka, Professor for the Physics of Energy Conversion and Storage at the TUM.
Working together with his team, the chemist has now provided a crucial prerequisite for doing so: For the first time, a scanning tunneling microscope has successfully been used to examine the surface during a catalytic process. In this manner, it was possible to determine in detail the locations at which the reaction speed and hence the activity of the catalysts is the highest. The findings have been published in the journal "nature".
On the search for active centers
For a long time now, researchers have suspected that there is a relationship between the surface structure and the activity of heterogeneous catalysts, where chemical reactions take place at the boundary surface between a solid and a liquid or gas. Heterogeneous catalysts are used for example in the electrolytic production of hydrogen gas or for cleaning vehicle exhaust gases.
"However, the chemical reactions do not take place at the same speed at all locations. Instead, there are active centers on the surface of the catalysts", reports Bandarenka. "Previously, we had to rely on model calculations and indirect measurements to localize these centers."
With the new analysis procedure, the existence of the active centers can now be proven experimentally. Samples with catalyst materials — including platinum and a combination of gold and palladium — are covered with a liquid electrolyte layer and examined using a scanning tunneling microscope.
While hydrogen ions (i.e. protons) receive electrons from the electrode at the surface of the catalyst and form hydrogen gas, the tip of the microscope scans the surface of the catalyst at a distance of just a few angstroms. Point for point, the "tunneling current" which flows between the surface and the tip is now measured. A computer connected to the device registers the signals.
A "noisy" mystery
"Interestingly, the tunneling currents are not the same everywhere. There are areas where the current is stronger, but flows unevenly – it is 'noisy' ", reports Bandarenka. The existence of this noise has been known for a long time, but to date, nobody had investigated what causes it.
During the evaluation of the data, the TUM team discovered a distinct relationship between the intensity of the noise and defects on the surface of the catalysts – microscopically small steps, edges, or corners. "As the number of defects increases, so does the noise – more electrons flow and hence more current as well", explains Bandarenka.
The fast food principle
The researcher likes to compare the behavior of the ions with that of guests at a fast food restaurant. When they are only able to find uncomfortable seating, they leave right away without consuming anything. On the other hand, if the seats are exceedingly comfortable, they remain seated for a long time and occupy the seating, blocking it for new guests. It is only when the seating is neither too comfortable nor too uncomfortable that customers come, eat, and leave again.
Viewed in terms of the chemical processes during electrolysis, this means the following: If the surface of the catalyst is too chemically attractive or repellent for the hydrogen ions, the reaction breaks down. The most effective areas are where ions are attracted, but do not remain for too long.
Fewer neighbors make for better reactions
Small defects in the atomic lattice, but also borders between materials — for example palladium on gold — appear to create these ideal conditions for catalysis. But why? "Our experiments show that the number of neighboring atoms and the resulting strength of the bond is a crucial factor for activity", explains Oliver Schneider, one of the co-authors of the publication.
The TUM researchers now intend to use the findings to develop more effective catalytic materials with active areas that are as large as possible.