A new synthesis route for alternative catalysts of noble metals
Researchers have developed a new synthesis route for alternative catalysts of noble metals for versatile chemical reactions that could help address environmental concerns.
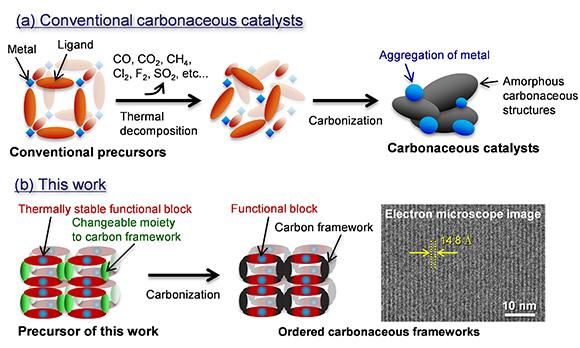
Synthesis schemes of (a) conventional carbonaceous catalysts and (b) this work for ordered carbonaceous frameworks.
Hirotomo Nishihara
Noble metals such as platinum are useful as catalysts for versatile chemical reactions including fuel cell vehicles and reduction of CO2 emission. However, they are too costly to be used for these purposes.
As inexpensive alternatives, organic-based catalysts and carbonaceous catalysts were explored, but were ultimately found to be impractical. This was because organic-based catalysts tend to be active but unstable, while carbonaceous catalysts are stable but less active.
In this work, researchers believe they have found a solution by developing a new synthesis route for intermediate materials of organic-based catalysts and carbonaceous catalysts.
While conventional carbonaceous catalysts have amorphous carbonaceous structures that cause a decline in catalytic activities (Fig. 1a), the new synthesis route enables the formation of carbonaceous catalysts with controlled chemical structures like organic-based catalysts (Fig. 1b). This synthesis route is capable of developing alternative catalysts of noble metals for many eco-friendly technologies such as fuel cell vehicles, hydrogen generation from water and CO2 reduction.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.


























































