Selective electrocatalysts to boost direct methanol fuel cell performance
Advertisement
A research group from the Institute of process engineering (IPE), Chinese Academy of Sciences recently reported the development of a new technology to boost performance of direct Methanol fuel cells (DMFCs) using high-concentration methanol as fuel, shedding some light on the design of clean and affordable alternative energy sources for portable electric devices.
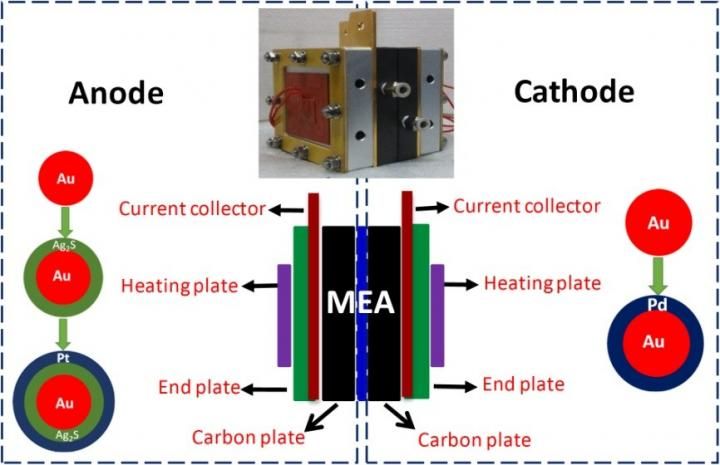
These are DMFC assemblies. Schematic illustration showing a DMFC fabricated with selective electrocatalysts at the anode and cathode chambers. Inlet is the photograph of the assembled cell.
Image by YANG Jun
When methanol, the fuel of DMFCs, crosses over from the anode to the cathode through the proton exchange membrane (PEM), fuel cell performance is significantly degraded, creating a major problem for the commercialization of DMFCs. Commonly, scientists use various strategies to improve DMFC performance at high concentrations of methanol. These include improving the fuel-feed system, membrane development, modification of electrodes, and water management.
"These conventional strategies do not fundamentally overcome the key obstacle, but inevitably complicate the design of DMFCs and hence increase their cost," said Yang Jun, an IPE professor. Working with Feng Yan, a doctoral student, and Liu Hui, an assistant professor, Yang used selective electrocatalysts to run a DMFC at methanol concentrations up to 15 M, an alternative method for solving the methanol crossover in DMFCs.
The anode and cathode catalysts of DMFCs are commonly based on platinum (Pt). These catalysts are not selective for the methanol oxidation reaction (MOR) at the anode or the oxygen reduction reaction (ORR) at the cathode. With a deep understanding of the mechanisms of electrode reactions in DMFCs, the researchers designed and produced noble metal-based heterogeneous electrocatalysts with enhanced catalytic activity and high selectivity for MOR and ORR.
Encouragingly, the DMFCs operated extremely well with high-concentration methanol as fuel by sufficiently making use of the structural uniqueness and electronic coupling effects among the different domains of the noble metal-based heterogeneous electrocatalysts.
As shown in Fig. 1, ternary Au-Ag2S-Pt nanocomposites with core-shell-shell structures display superior anode selectivity due to the electronic coupling among their different domains, while core-shell Au-Pd nanoparticles with thin Pd shells exhibit excellent cathode selectivity because of the synergistic effects between their Au core and thin Pd shell.
The as-fabricated DMFC with selective catalysts produces a maximum power density of 89.7 mW cm-2 at a methanol-feed concentration of 10 M, and maintains good performance at methanol concentrations up to 15 M.
"Next, we are going to optimize the overall size of the catalysts, e.g., using Au nanoclusters with fine diameters as starting materials to further enhance the activity/selectivity for DMFC reactions," said YANG. In this way, new technologies will be created to help improve the design of more cost-effective and efficient DMFC systems.