Control of material crystallization by agitation
The transition of unstructured amorphous materials into structured crystalline materials is generally induced by heating materials above their transition temperature. Crystalline materials are important in technology like devices, so alternative ways to control their formation has attracted much interest from materials scientists. Researchers have found that crystallization can be facilitated at a lower temperature than the traditional transition temperature if an amorphous material is agitated at a certain frequency. Thus, agitation represents a possible alternative to temperature for controlling the crystallization of materials.
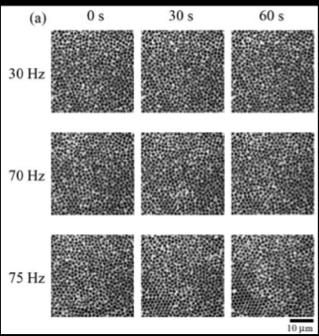
Representative experimental results are shown. Crystallization hardly occurs by mechanical oscillations at 30 and 70 Hz, but 75 Hz oscillation causes crystallization.
N. Nakamura, K. Inayama, T. Okuno, H. Ogi, and M. Hirao, Scientific Reports 7, 1369 (2017), doi:10.1038/s41598-017-01484-y
A team at Osaka University decided to clarify the relationship between the agitation and crystallization of amorphous solids. To aid their investigation, they used a colloidal (small particle) system to model atomic materials because the larger particle size and relaxation time of colloids, compared with those of atoms, facilitate their measurement.
"We prepared colloidal glasses from silica spheres in solution and then oscillated them at different frequencies," explains first author Nobutomo Nakamura. "We then observed the resulting structure by confocal laser scanning microscopy."
The group identified a specific frequency at which crystallization of their system was accelerated. They determined the degree of crystallization in the system agitated at different frequencies by measuring its local bond orientational order parameter. The value of this parameter increased considerably, indicating a higher degree of crystallization, only when the system was agitated at a frequency of around 75 Hz.
"Our results indicated there is a specific vibrational mode that facilitates crystallization of the colloid," says Nakamura.
The researchers then confirmed that the frequency at which crystallization occurs changed depending on the interaction between particles in the system. They added a polymer to the system to alter the interaction force between particles, which caused the crystallization frequency to increase. The team were able to explain their findings by relating the crystallization frequency with the time scale of the vibrational motion of the particles. They proposed that agitation of the system at a frequency that matched the motion of particles forming crystalline structure aided their collective movement and thus accelerated crystallization.
These findings reveal that it may be possible to control the crystallization of amorphous systems by agitation at a specific frequency rather than heating above their transition temperature. This may allow the formation of crystalline materials at a lower temperature, which will be useful in device manufacturing.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.