Next-gen solar cells could be improved by atomic-scale redesign
Researchers have uncovered the exact mechanism that causes new solar cells to break down in air, paving the way for a solution.
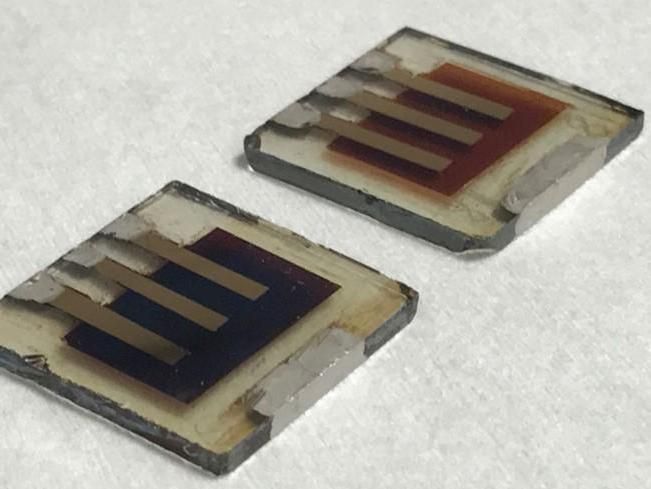
Fresh (L) and degraded (R) solar cells are pictured.
Imperial College London
Solar cells harness energy from the Sun and provide an alternative to non-renewable energy sources like fossil fuels. However, they face challenges from costly manufacturing processes and poor efficiency - the amount of sunlight converted to useable energy.
Light-absorbing materials called organic lead halide perovskites are used in a new type of solar cells that have shown great promise, as they are more flexible and cheaper to manufacture than traditional solar cells constructed of silicon.
However, perovskite cells degrade rapidly in natural conditions, greatly decreasing their performance in a matter of days. This is one reason they are not currently widely used.
Previously, a team led by scientists from the Department of Chemistry at Imperial discovered that this breakdown is due to the formation of ‘superoxides’ that attack the perovskite material. These superoxides are formed when light hitting the cells releases electrons, which react with the oxygen in the air.
Now, in a study published in Nature Communications , the team have determined how the superoxides form and how they attack the perovskite material, and have proposed possible solutions.
Not-so-super oxides
Working with Dr Christopher Eames and Professor Saiful Islam at the University of Bath, the team found that superoxide formation is helped by spaces in the structure of the perovskite normally taken up by molecules of iodide. Although iodide is a component of the perovskite material itself, there are defects where iodide is missing. These vacant spots are then used in the formation of superoxides.
The team found that dosing the material with extra iodide after manufacturing did improve the stability, but that a more permanent solution could be to engineer the iodide defects out.
Lead author of the new study, Nicholas Aristidou from the Department of Chemistry at Imperial, said: “After identifying the role of iodide defects in generating superoxide, we could successfully improve the material stability by filling the vacancies with additional iodide ions. This opens up a new way of optimising the material for enhanced stability by controlling the type and density of defects present.”
Lead researcher Dr Saif Haque from the Department of Chemistry at Imperial added: “We have now provided a pathway to understand this process at the atomic scale and allow the design of devices with improved stability.”
Better solutions
Currently, the only way of protecting perovskite cells from degradation by air and light is to encase them in glass. However, perovskite solar cells are made from flexible material designed to be used in a range of settings, so the glass encasement severely limits their function.
Dr Haque said: “Glass encasement restricts movement and adds weight and cost to the cells. Improving the perovskite cell material itself is the best solution.”
The team hope to next test the stability of the cells in real-world settings. The cells would be exposed to a combination of both oxygen and moisture, testing the cells in more relevant scenarios.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.