Two-dimensional melting of hard spheres experimentally unravelled after 60 years
First definitive experimental evidence of two-dimensional melting of hard spheres
After extensive research, scientists from the Department of Chemistry at the University of Oxford have found experimental evidence that sheds new light on the melting of two-dimensional substances. Findings from the study could be used to support technological improvements to thin film materials such as graphene.
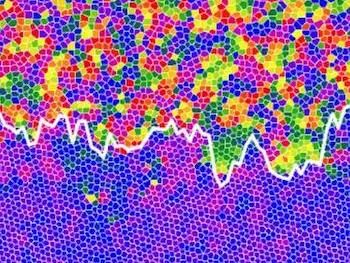
This is the interface between the liquid (top) and hexatic (bottom) states.
Oxford University
Researchers from the group of Professor Roel Dullens at Oxford's Department of Chemistry have experimentally elucidated how melting of a two-dimensional solid of hard spheres occurs. With this work they resolve one of the most fundamentally important but still outstanding issues in condensed matter science. In addition, these results provide the cornerstone for the further understanding and development of two-dimensional materials.
Melting, the phase transition in which a substance turns from a solid to a liquid, is widely understood in basic terms. But despite being encountered regularly in everyday life, (whether in the workplace, home or natural world), scientists have long been trying to understand the melting process on a fundamental level.
The melting of a solid into a liquid is one of the most commonly experienced scientific phenomena. However, understanding this transformation is especially mysterious for solids in two-dimensions. Here, the celebrated Kosterlitz-Thouless-Halperin-Nelson-Young (KTHNY) theory proposes that an intermediate, partially disordered state, called the 'hexatic', exists between the solid and liquid. Substantial effort has been made towards the understanding of these 'topological' transitions, for which Kosterlitz and Thouless were awarded the 2016 Nobel Prize in Physics. Yet for the simplest interacting system of many particles, two-dimensional hard spheres, there has been an astonishing lack of consensus despite the first simulations being performed over 60 years ago.
Dr Alice Thorneywork and co-workers used optical microscopy to study monolayers of colloidal model hard spheres tilted by a small angle to introduce a gradient in the particle concentration. For hard spheres, the behaviour is governed only by this concentration, which allowed them to identify and characterize the liquid, hexatic, and solid states and the nature of the transitions between them in a single experiment. The results show that the melting occurs via a continuous solid-hexatic transition followed by a first order hexatic-liquid transition.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

New Study Sheds Light on the Hidden World of Solvation Shells - Groundbreaking discovery
Dow Europe Announces Price Increase for Polyethylene, Polypropylene and Polystyrene Resins