Coordination chemistry of anions through halogen-bonding interactions
Advertisement
While an IUPAC definition of hydrogen bonding was only released in 2011 after decades of discussions in the scientific community, it did not take such a long time to come up with an analogous definition of halogen bonding, following a revival of this interaction in the literature which can be traced back to the early 1990s, Fourmigué, M. (2017). Acta Cryst. B73, 138-139
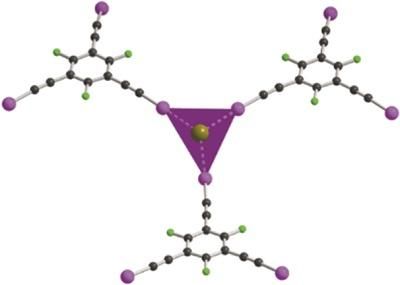
This image shows the detail of the coordination spheres of the bromide anion with Et3BuN+Br-
M. Fourmigué
The halogen-bonding interaction is essentially described as an electrostatic interaction between a charge concentration (Lewis base) and a charge-depleted area, called an σ-hole, that a covalently bound halogen atom exhibits in the extension of this bond.
In a recent paper by Szell et al, (2017). Acta Cryst. B73, 153-162 single-crystal X-ray diffraction structures have been reported for a series of seven halogen-bonded co-crystals featuring 1,3,5-tris(iodoethynyl)-2,4,6-trifluorobenzene as the halogen-bond donor, and bromide ions (as ammonium or phosphonium salts) as the halogen-bond acceptors. Depending on the stoichiometry, the resulting frameworks can form honeycomb structures of variable geometry, but also systems with four or six halogen bonds to the bromide ion. While the counter-cations generally occupy the void spaces in the present work, the construction of halogen-bonded frameworks with potential gas storage applications is an appealing prospect which may be facilitated in the future by ligands enabling directional and multidentate interactions.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Wake_turbulence
Sodium_fusion_test

Improve your practical know-how of polymer analysis - Application Notebook for Efficient Polymer Analysis using GPC/SEC
Acid-base_extraction
The Fading Memory of Science: ESF joins alliance to preserve science assets of the digital age
RET_proto-oncogene
Platelet-derived_growth_factor_receptor
Bone_morphogenetic_protein
Vascular_endothelial_growth_factor
Chlorite
Glufosinate