Discovery of 'helical molecular glue'
Advertisement
The research group led by Professor Hideto Tsuji conducts basic and applied researches on biodegradable polymers derived from renewable resources such as corn or potato starch. The group mainly studies a typical biodegradable polymer poly(lactic acid). Poly(lactic acid) is hydrolyzed and degraded in the human body and the resulting lactic acid is metabolized without causing adverse effects to the body. Because of this advantage, poly(lactic acid) is used in medical applications as a scaffold material for tissue regeneration and also in environmental applications.
This image shows helical molecular glue.
Tsuji, H. et al. Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers. Sci. Rep. 7, 45170; doi: 10.1038/srep45170 (2017).
These are molecular structures of unsubstituted and substituted poly (lactic acid).
Tsuji, H. et al. Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers. Sci. Rep. 7, 45170; doi: 10.1038/srep45170 (2017).
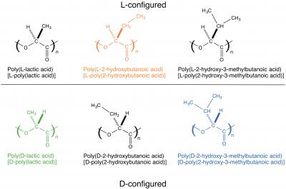
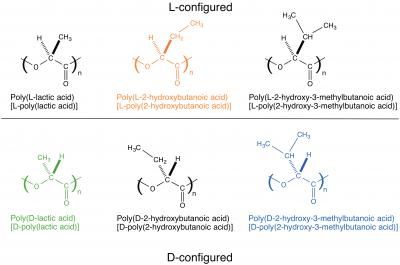
Poly(lactic acid) contains an asymmetric carbon and therefore occurs either as the L- or D-enantiomer, namely poly(L-lactic acid) or poly(D-lactic acid). Since the interaction between different enantiomers (i.e. between L and D) is stronger than that between the same enantiomers (e.g. between D and D), blending the two enantiomers results in co-crystallization of an L-enantiomer and a D-enantiomer (this phenomenon is also called stereocomplex formation). The stereocomplex has a higher melting point, better mechanical properties, and higher heat resistance and hydrolysis resistance than those of their constituent enantiomers, and therefore the stereocomplex can have wider applications than those of conventional biodegradable materials. Under these circumstances, stereocomplex formation between poly(lactic acid) has been actively researched in recent years.
L-poly(lactic acid) is counterclockwise-helical, and D-poly(lactic acid) is clockwise-helical. Therefore, the fact that L-poly(lactic acid) and D-poly(lactic acid) form a stereocomplex together indicates that a counterclockwise-helical molecule and a clockwise-helical molecule are strongly attracted to each other. Tsuji et al. have also discovered that blending the L- and D-enantiomers of poly(2-hydroxybutanoic acid) (a poly(lactic acid) with its methyl group replaced by an ethyl group) results in stereocomplex formation as well. In addition, there are reports on the same phenomena occurring to poly(2-hydroxy-3-methylbutanoic acid) (a poly(lactic acid) with its methyl group replaced by an isopropyl group) and occurring even between poly(lactic acid) with different side chains (for example, between L-poly(lactic acid) and D-poly(2-hydroxybutanoic acid)). All these phenomena indicate the presence of strong interaction between a counterclockwise-helical molecule and a clockwise-helical molecule.
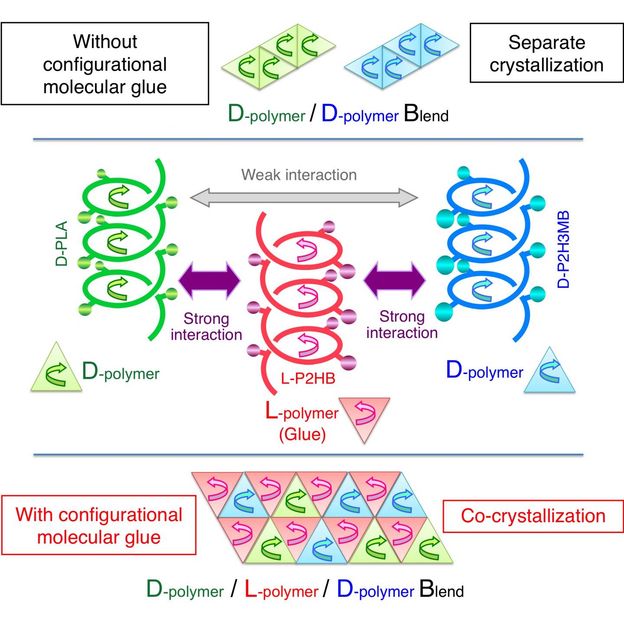
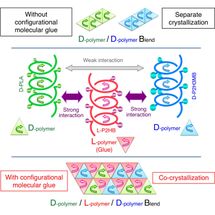
This time, Tsuji et al. have found the action of a counterclockwise-helical molecule to glue two structurally-different clockwise-helical molecules that do not bind to each other otherwise. This finding indicates that a clockwise-helical molecule would also have the action to glue two structurally-different counterclockwise-helical molecules that do not bind to each other otherwise. Through experiment using D-poly(lactic acid), L-poly(2-hydroxybutanoic acid), and D-poly(2-hydroxy-3-methylbutanoic acid), Tsuji et al. have discovered for the first time worldwide that counterclockwise-helical L-poly(2-hydroxybutanoic acid) acts as "helical molecular glue" to glue clockwise-helical D-poly(lactic acid) and clockwise-helical D-poly(2-hydroxy-3-methylbutanoic acid) and thereby co-crystallizes these two D-molecules despite that these two do not usually co-crystalize. This finding has opened the door to binding various polymers that are coiled in the same direction. Now that the degree of freedom in polymer combination has increased, development of new polymer materials with various properties has become possible.




























































