Rare Earths Become Water-repellent Only as They Age
Surfaces that have been coated with rare earth oxides develop water-repelling properties only after contact with air. Even at room temperature, chemical reactions begin with hydrocarbons in the air. In the journal Scientific Reports, researchers from the University of Basel, the Swiss Nanoscience Institute and the Paul Scherrer Institute report that it is these reactions that are responsible for the hydrophobic effect.
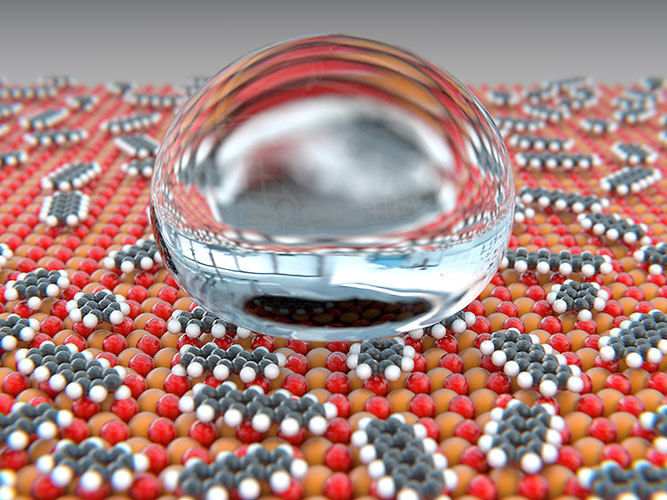
Rare earth oxides (red/orange) react with gaseous organic compounds from the ambient air to form carbonates and hydroxides (grey/white). Through this reaction, the surface develops water-repelling properties.
University of Basel, Department of Physics
Rare earths are metals found in rare earth minerals. They are used today in, among other things, automotive catalytic converters and batteries, in the production of screens and lamps, and as a contrast agent in magnetic resonance imaging. Their broad range of applications means that there is a high demand for rare earths, and this demand is constantly increasing.
Additional uses for rare earths were opened up after American researchers reported in 2013 that surfaces that have been coated with rare earth oxides become water-repellent.
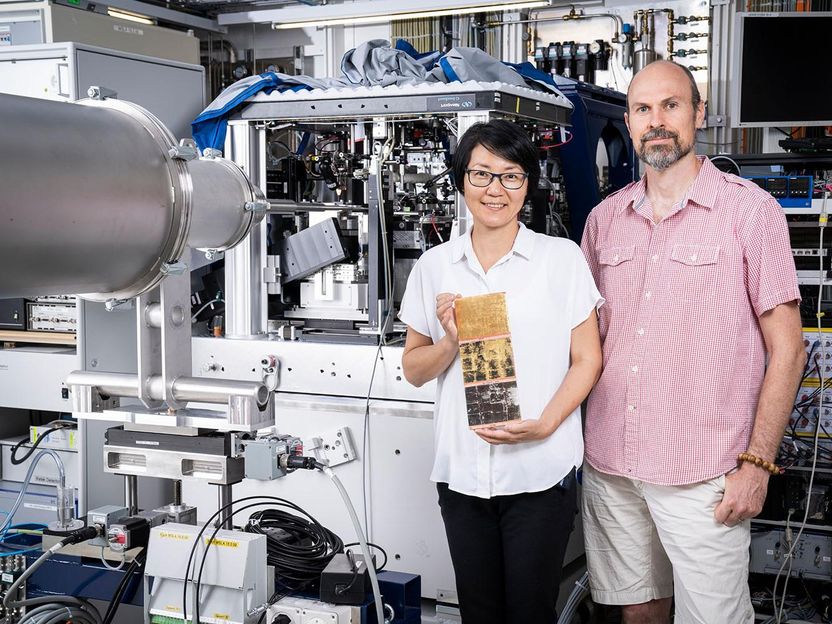
Scientists from the University of Basel, the Swiss Nanoscience Institute and the Paul Scherrer Institute have now worked with the company Glas Trösch to examine these hydrophobic properties more closely.
Water-repellency develops only after chemical reactions
The researchers coated glass pieces with rare earth oxides, nitrides and fluorides and analyzed how well they could be wetted with water.
They could not detect any hydrophobic properties when the coating was freshly deposited. It was only chemical reactions with gaseous hydrocarbons found in the ambient air that increased the surfaces’ roughness and reduced wetting by water.
The gaseous organic compounds from the ambient air are first adsorbed by the surface and then react with the oxides to form carbonates and hydroxides until the surface is completely covered with these compounds. This process takes place even at room temperature.
“We were surprised that the hydrophobic effect was caused by the surface aging,” says Professor Ernst Meyer, from the Department of Physics at the University of Basel, commenting on the results of the project supported by the Commission for Technology and Innovation (CTI). These conclusions are very revealing from a scientific point of view because catalytic processes also frequently take place at room temperature, which makes it important to understand the surface’s physical properties.
The examined materials are, however, unsuitable for the industrial production of water-repellent glass surfaces, because the glass requires a sophisticated storage process before it shows the desired hydrophobic characteristics.
Original publication
Elçin Külah, Laurent Marot, Roland Steiner, Andriy Romanyuk, Thomas A. Jung, Aneliia Wäckerlin, & Ernst Meyer; "Surface chemistry of rare-earth oxide surfaces at ambient conditions: reactions with water and hydrocarbons"; Scientific Reports; 2017
Most read news
Original publication
Elçin Külah, Laurent Marot, Roland Steiner, Andriy Romanyuk, Thomas A. Jung, Aneliia Wäckerlin, & Ernst Meyer; "Surface chemistry of rare-earth oxide surfaces at ambient conditions: reactions with water and hydrocarbons"; Scientific Reports; 2017
Topics
Organizations
Other news from the department science
These products might interest you
Dursan by SilcoTek
Innovative coating revolutionizes LC analysis
Stainless steel components with the performance of PEEK - inert, robust and cost-effective
OCA 200 by DataPhysics
Using contact angle meter to comprehensively characterise wetting behaviour, solids, and liquids
With its intuitive software and as a modular system, the OCA 200 answers to all customers’ needs
Tailor-made products for specific applications by IPC Process Center
Granulates and pellets - we develop and manufacture the perfect solution for you
Agglomeration of powders, pelletising of powders and fluids, coating with melts and polymers

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Nanofarming technology harvest biofuel oils without harming algae - Ames Laboratory and Catilin seek to commercialize new algal oil extraction process
Oxea Announces Price Increase for Carboxylic Acids
Sharp X-ray images despite imperfect lenses - New method for X-ray microscopy

Scientists devise catalyst that uses light to turn carbon dioxide to fuel
Highly promising solid electrolytes for high-performance lithium-ion batteries