Revolutionary process to create ether from esters using metal catalysts
A group of Waseda University researchers has developed a new process using palladium or nickel as a catalyst for removing carbon monoxide from esters to produce ethers. This innovation provides new opportunities for development of drugs to fight cancer, malaria and more.
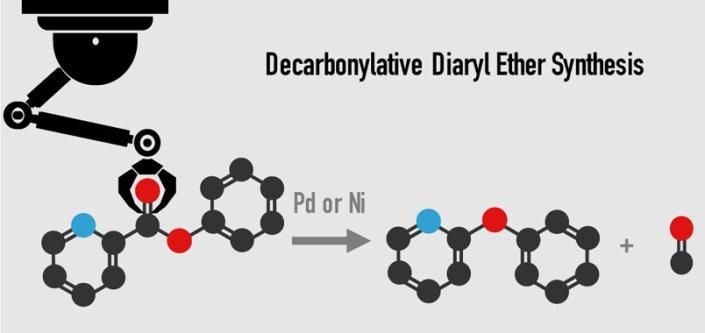
This is a better way to make diaryl ether.
Waseda University
The conventional method for producing diaryl ether uses an intermolecular cross-coupling reaction of aryl halides and phenols with a copper or palladium catalyst, but high cost and concerns about disposal of potentially hazardous halogenated waste have driven demand for a better method.
In this research, a nickel or palladium catalyst with an enabling diphosphine ligand successfully removed carbon monoxide from aromatic esters to synthesize diaryl ether. Using this innovative process, diaryl ethers can be produced from over 30 different kinds of aromatic esters, allowing a choice of more inexpensive and easily obtainable materials. The present reaction can also be conducted on a gram scale with excellent yield, all of which is expected to make a significant impact on development of new pharmaceuticals.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.
Last viewed contents
Neon_lamp

Go with the flow: Scientists design new grid batteries for renewable energy
Satellite_laser_ranging
Self-consistent_mean_field_(biology)
Phalaris_(grass)
Vion_Pharmaceuticals,_Inc.

Using a microscopic ring to produce pulsed light
Gamma_ray

Scientists show how to store liquid fuels in polymeric gels to prevent explosions and fires - Researchers from Japan investigate a new safer way to transport and store fuels
Yara acquires remaining shares in Yara Nipro
Game-changing technology to remove 99% of carbon dioxide from air - Carbon capture advance could bring environmentally friendly fuel cells closer to market




























































