Getting Rid of the Last Bits of Sulfur in Fuel
Scientists led by a team at Caltech have developed a new method for potentially removing nearly all sulfur compounds from gas and diesel fuel.
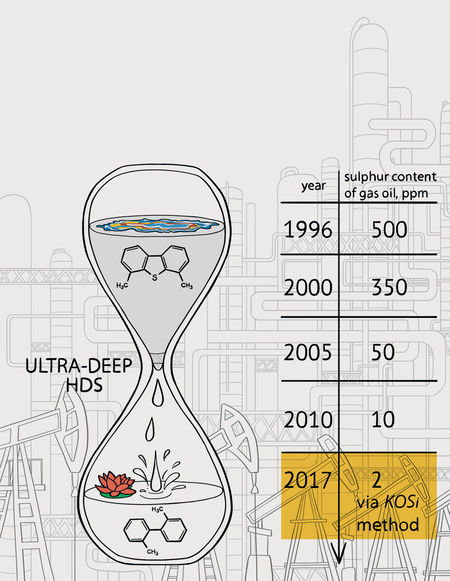
The ability to reduce sulfur in fuels is an important step toward reducing emissions. However, extremely low levels can be difficult to achieve. Now, Caltech researchers have demonstrated an unorthodox approach that lowers sulfur content in diesel to about 2 ppm. The method uses Earth-abundant materials (potassium (K), oxygen (O), and silicon (Si)—hence the name, "KOSi") and operates under mild conditions.
Polina Nesterova
Sulfur compounds in fuels such as gasoline and diesel create air pollution when the fuel is burned. To address that challenge, large-scale oil refinery processes remove the majority of sulfur from fuel down to a government-mandated level. The new technique, however, has the potential to reduce sulfur down to a fraction of that amount, which would further reduce air pollution and extend the lifetime of vehicles' catalytic converters, which control tailpipe emissions.
The results—from a team led by Caltech and BP, and in collaboration with researchers at UCLA, ETH Zürich, and China's Nanjing University—are described in a new study.
"We simulated a high-sulfur oil and eliminated almost all of the sulfur through a simple chemical process. The next step is figuring out how to streamline the process and make it work on an industrial scale," says lead author Anton Toutov, a graduate student in the lab of Robert Grubbs , the Victor and Elizabeth Atkins Professor of Chemistry at Caltech.
The new method uses a potassium salt to induce the chemical reactions required to remove sulfur from fuel. Potassium is an abundant element on Earth and cheaper and more environmentally friendly to use than rare metal catalysts that are used for similar reactions.
"We were really surprised how well the potassium salt worked," says Toutov, who is also a Dow-Resnick Fellow at the Resnick Sustainability Institute at Caltech. "The sulfur is contained in small organic molecules, and this process just rips it right out."
The discovery that potassium salts can be used to promote key chemical reactions came unexpectedly a couple of years ago. Researchers in the Grubbs laboratory had been testing ways to break carbon-oxygen bonds, which is most efficient when done with a precious metal catalyst such as platinum. Alexey Fedorov of ETH Zürich, who was a postdoctoral fellow in the Grubbs laboratory at the time, ran a control experiment without the metal catalyst and found that the reaction still worked. After several tests, the researchers confirmed that a potassium salt, called potassium tert-butoxide, was, in fact, driving the reaction. Next, Toutov optimized the process and further showed that the reaction produced compounds with carbon-silicon bonds , which normally require metal catalysts to form. Carbon-silicon bonds are found in many products, such as polymers, agricultural chemicals, and semiconductors.
"They left the metal out of the reaction, and it still worked," says Grubbs. "This was a huge surprise."
Toutov and his colleagues in the Grubbs lab have used the potassium salt method to remove sulfur from carbon compounds found in diesel fuel. They partnered with BP to test their method on the company's refined diesel samples, reducing the sulfur levels down from 8 parts per million (comparable to the highest quality of diesel you can get from a typical gas pump today) to an extremely low 2 parts per million. They also repeated the experiment with diesel spiked with high levels of sulfur and achieved similar results.
The new method could be used as an additional step in the oil refinement process to get rid of the last traces of sulfur in fuels. The next step for Toutov, who is co-founding a new company, Fuzionaire, is to commercialize this technology. "We have a number of ideas in mind on how to do that," he says, including recycling waste products from other industries for use in the process.
Original publication
Toutov, Anton A. and Salata, Mike and Fedorov, Alexey and Yang, Yun-Fang and Liang, Yong and Cariou, Romain and Betz, Kerry N. and Couzijn, Erik P. A. and Shabaker, John W. and Houk, Kendall N. and Grubbs, Robert H.; "A potassium tert-butoxide and hydrosilane system for ultra-deep desulfurization of fuels"; Nature Energy; 2017




























































