Seeking 'game-changer' in electrochemical water catalysis
Advertisement
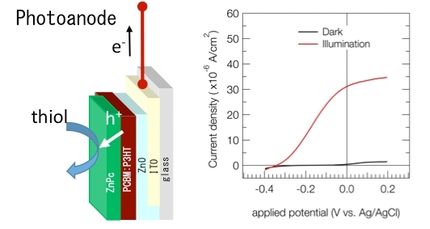
The world's current reliance on fossil fuel energy, which is finite and a major source of pollution, begs for new, greener energy solutions. Electrochemical water catalysis or "water splitting," the chemical reaction by which water is separated into oxygen and hydrogen, is one means of tapping energy from such renewable energy sources as solar and wind. But development of efficient and cost-effective methods to achieve commercial-scale water splitting remains a holy grail of science, says Utah State University chemist Yujie Sun.
"Splitting water molecules is simple on an experimental basis, but difficult and expensive on a large-scale basis," says the assistant professor in USU's Department of Chemistry and Biochemistry. "Two half reactions are involved in the whole process of water splitting. One is water oxidation to generate oxygen and the other is water reduction to produce hydrogen."
The sluggish kinetics of water oxidation remains a vexing bottleneck for widespread, affordable hydrogen production from sustainable energy inputs, he says. "Ironically, the product of oxygen from water oxidation is not of significant value."
Sun, the 2017 recipient of a prestigious Faculty Early Career Development 'CAREER' Award from the National Science Foundation, is investigating potentially game-changing ways, at the molecular level, of surmounting existing roadblocks. The NSF's highly competitive grant program for junior faculty, CAREER awards recognize demonstrated excellence in research, teaching and the integration of education and research. The award provides Sun with a five-year, $592,791 grant to pursue his research, while inspiring a new generation of scientists.
"Researchers in my lab range from high school students to postdoctoral fellows," says Sun, who joined USU's faculty in 2013. "Together, we're looking beyond current knowledge to create novel electrolyzers, by employing inexpensive catalysts for hydrogen production at much lower energy input compared to those of conventional water-splitting processes."
Sun is exploring a new oxidative process that would produce value-added organic products in the liquid phase, while simultaneously providing electrons for the production of hydrogen, which would be released in the gas phase.
"In this case, no potentially hazardous hydrogen-oxygen mixing would arise," he says. "The result would be a safer, easier-to-transport product and a clean source of fuel."
In particular, Sun is investigating the development of a biomass intermediate for the oxidation half reaction, which could provide a green, water-soluble polymer precursor to replace such fossil fuel-derived polymers as polyethylene terephthalate or 'PET.' PET is used in all manner of fabrics, household products, furnishing, appliances, vehicle interiors and more.
"This could be very valuable," he says. "Imagine all the products around you that could be made from the clean and renewable energy sources like sun and wind."
As part of his project, Sun will teach an inorganic intermediate lab for undergraduates that incorporates new, research-based experiments.
"We're replacing old-school techniques with cutting-edge research with an emphasis in green chemistry," he says.
Sun is continuing his involvement with USU Eastern Blanding Campus's Native American STEM Mentorship Program, which provides students opportunities to get involved in undergraduate research at USU's Logan campus, including Sun's Lab. In addition, Sun is mentoring high school students as part of the American Chemical Society's Project SEED, a summer research internship program for economically disadvantaged teens.
"This project allows high school students to work alongside mentors in our lab and gain insight about college and future career paths," he says. "Some of these students will have opportunities to present their research and publish in professional journals."
To share knowledge across Utah's campuses, Sun is also continuing his association with the annual Utah Inorganic/Organometallic Symposium, a yearly summer gathering he initiated with colleagues from the University of Utah and Brigham Young University.
"We've found this is a great way to share information, foster ideas and collaborate with groups around the state," he says. "It's a wonderful networking opportunity for students to gain confidence, as they present their efforts to others."