New form of matter unveiled: Time crystals
To most people, Crystals mean diamond bling, semiprecious gems or perhaps the jagged amethyst or quartz crystals beloved by collectors.
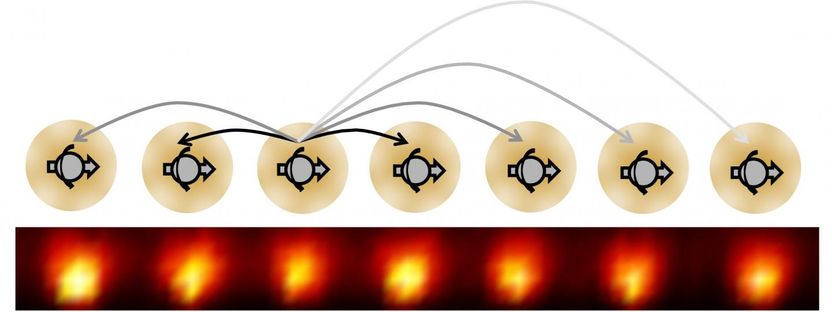
Following a blueprint created by UC Berkeley physicist Norman Yao, physicists at the University of Maryland made the first time crystal using a one-dimensional chain of ytterbium ions. Each ion behaves like an electron spin and exhibits long-range interactions indicated by the arrows.
Chris Monroe, University of Maryland
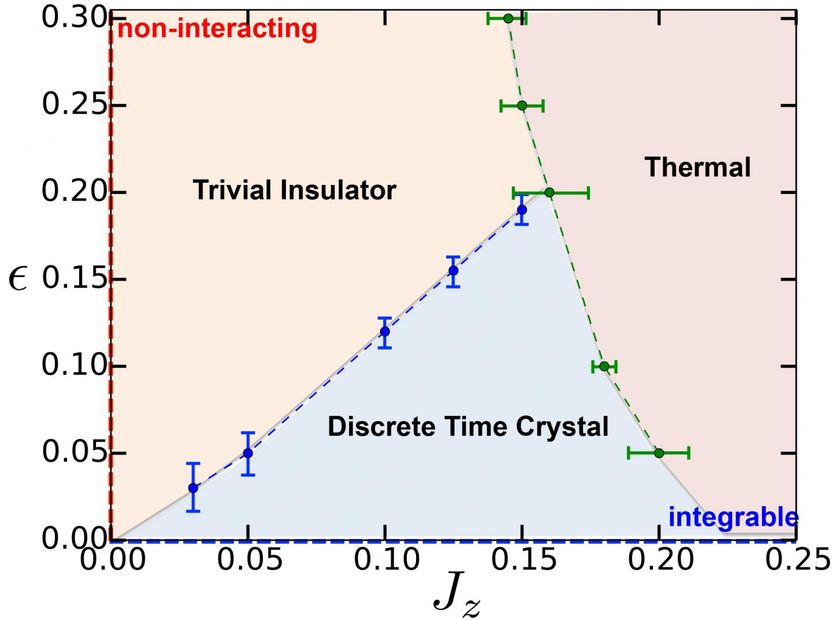
This phase diagram shows how changing the experimental parameters can 'melt' a time crystal into a normal insulator or heat up a time crystal to a high temperature thermal state.
Norman Yao, UC Berkeley
To Norman Yao, these inert crystals are the tip of the iceberg.
If crystals have an atomic structure that repeats in space, like the carbon lattice of a diamond, why can't crystals also have a structure that repeats in time? That is, a time crystal?
The University of California, Berkeley assistant professor of physics describes exactly how to make and measure the properties of such a crystal, and even predicts what the various phases surrounding the time crystal should be -- akin to the liquid and gas phases of ice.
This is not mere speculation. Two groups followed Yao's blueprint and have already created the first-ever time crystals. The groups at the University of Maryland and Harvard University reported their successes, using two totally different setups, in papers posted online last year, and have submitted the results for publication. Yao is a co-author on both papers.
Time crystals repeat in time because they are kicked periodically, sort of like tapping Jell-O repeatedly to get it to jiggle, Yao said. The big breakthrough, he argues, is less that these particular crystals repeat in time than that they are the first of a large class of new materials that are intrinsically out of equilibrium, unable to settle down to the motionless equilibrium of, for example, a diamond or ruby.
"This is a new phase of matter, period, but it is also really cool because it is one of the first examples of non-equilibrium matter," Yao said. "For the last half-century, we have been exploring equilibrium matter, like metals and insulators. We are just now starting to explore a whole new landscape of non-equilibrium matter."
While Yao is hard put to imagine a use for a time crystal, other proposed phases of non-equilibrium matter theoretically hold promise as nearly perfect memories and may be useful in quantum computers.
An ytterbium chain
The time crystal created by Chris Monroe and his colleagues at the University of Maryland employs a conga line of 10 ytterbium ions whose electron spins interact, similar to the qubit systems being tested as quantum computers. To keep the ions out of equilibrium, the researchers alternately hit them with one laser to create an effective magnetic field and a second laser to partially flip the spins of the atoms, repeating the sequence many times. Because the spins interacted, the atoms settled into a stable, repetitive pattern of spin flipping that defines a crystal.
Time crystals were first proposed in 2012 by Nobel laureate Frank Wilczek, and last year theoretical physicists at Princeton University and UC Santa Barbara's Station Q independently proved that such a crystal could be made. According to Yao, the UC Berkeley group was "the bridge between the theoretical idea and the experimental implementation."
From the perspective of quantum mechanics, electrons can form crystals that do not match the underlying spatial translation symmetry of the orderly, three-dimensional array of atoms, Yao said. This breaks the symmetry of the material and leads to unique and stable properties we define as a crystal.
A time crystal breaks time symmetry. In this particular case, the magnetic field and laser periodically driving the ytterbium atoms produce a repetition in the system at twice the period of the drivers, something that would not occur in a normal system.
"Wouldn't it be super weird if you jiggled the Jell-O and found that somehow it responded at a different period?" Yao said. "But that is the essence of the time crystal. You have some periodic driver that has a period 'T', but the system somehow synchronizes so that you observe the system oscillating with a period that is larger than 'T'."
Yao worked closely with Monroe as his Maryland team made the new material, helping them focus on the important properties to measure to confirm that the material was in fact a stable or rigid time crystal. Yao also described how the time crystal would change phase, like an ice cube melting, under different magnetic fields and laser pulsing.
The Harvard team, led by Mikhail Lukin, set up its time crystal using densely packed nitrogen vacancy centers in diamonds.
"Such similar results achieved in two wildly disparate systems underscore that time crystals are a broad new phase of matter, not simply a curiosity relegated to small or narrowly specific systems," wrote Phil Richerme, of Indiana University, in a perspective piece accompanying the paper published in Physical Review Letters. "Observation of the discrete time crystal... confirms that symmetry breaking can occur in essentially all natural realms, and clears the way to several new avenues of research."
Yao is continuing his own work on time crystals as he explores the theory behind other novel but not-yet-realized non-equilibrium materials.



































































