Transforming Carbon Dioxide into a Key Industrial Ingredient
Advertisement
carbon dioxide doesn’t have a very positive image, as it is generally only considered to be a greenhouse gas. But scientists involved in the German Government-funded eEthylen project expect their research to yield important insights into how CO2 can be efficiently converted into ethylene, thus enabling them to optimize electrochemical synthesis processes for large-scale industrial applications.

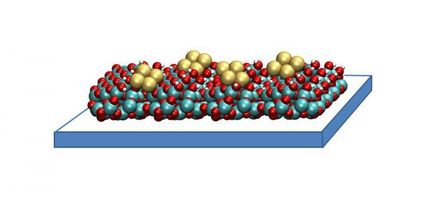
The use of an appropriate catalyst ensures the electrochemical synthesis of ethylene from carbon dioxide and water; otherwise electrolysis produces only hydrogen.
Siemens AG
“For us, carbon dioxide isn’t a waste product, but rather a valuable raw material with great prospects,” explains Dan Taroata, the project manager from the consortium leader, Siemens. In the eEthylen project, Taroata and his colleagues are working together with experts from Evonik, the Technical University of Berlin, Ruhr University Bochum, and the Helmholtz Institute Erlangen-Nürnberg to study how CO2 can be converted into ethylene. Taorata is convinced that CO2 will not only help to produce coveted materials but will also open up new business opportunities for Siemens.
With the help of a direct single-stage electrolysis system, the researchers are using electricity to synthesize ethylene out of carbon dioxide and water. Their work focuses on electro catalysts, because these materials can charge inert CO2 with its energy-rich electrons into ethylene. If the electrons instead congregate in the surrounding water, the process produces only hydrogen. That’s why the choice of which catalyst to use determines whether the method will be successful or not.
Putting Carbon Dioxide to Work
Together with their eEthylen partners, scientists from Siemens are now looking for a solution that will allow them to realize their vision. For the ethylene production process, Siemens is contributing a system from one of its key areas of expertise: an electrolysis facility for continuous operation. The system is based on electrolyzers for hydrogen production, which are already part of the Siemens product range.
The Technical University of Berlin is responsible for analyzing the system’s catalytic capacity and the range of chemical products, while Ruhr University Bochum is investigating the chemical composition of the catalysts. The Helmholtz Institute Erlangen-Nürnberg is contributing its electrode expertise, and Evonik is developing powder catalysts for the production of large electrolysis electrodes.
The three-year project, which was launched in October 2016, is part of the German government’s CO2Plus research initiative to investigate the use of CO2 in the production of raw materials. Siemens is the leader of the project consortium. Equipped with a total budget of €2.9 million, eEthylen could well revolutionize the manufacture of ethylene.
From Renewable Energy to Cheaper Ethylene
Traditional ethylene production methods are expensive and energy intensive. Typically, steam cracking is used – a process in which the petroleum distillate naphtha is heated to around 800–850 °Celsius until its long chains of hydrocarbons break down into short-chains. This process results in 15 different materials, which then have to be laboriously separated from one another. One of these materials is ethylene. But if an electrochemical process were used instead, the resulting ethylene would be economically attractive— in part because the price of solar and wind power is steadily falling.
Ethylene is currently used in a wide variety of ways. For one thing, it is the feedstock material for the production of polyethylene, polyvinylchloride, and polyester. As such, it is contained in most plastics. Ethylene also helps to make fruits and vegetables ripen at precisely the right time — an important application in a world of globalized food-supply chains.
If the eEthylen research team manages to optimize the electrolytic production process, the result could seriously challenge conventional manufacturing methods. And it could be a big money maker too. That’s because one ton of ethylene costs between €850 and €1,200 — a hefty sum, considering that around 180 million tons are used annually worldwide.