Hydrogen from sunlight - but as a dark reaction
Generation, storage, and time-delayed release of electrons in graphitic carbon nitride material for artificial photosynthesis
The storage of photogenerated electric energy and its release on demand are still among the main obstacles in artificial photosynthesis. One of the most promising, recently identified photocatalytic new materials is inexpensive graphitic carbon nitride. Scientists have now explored a modified form that can produce light-generated electrons and store them for catalytic hydrogen production even after the light has been switched off. They present this biomimetic photosynthesis approach in the journal Angewandte Chemie.
© Wiley-VCH
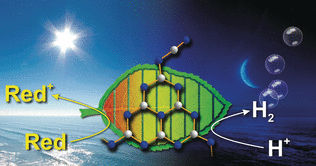
Nature has split photosynthesis into a light reaction generating electrons and holes from solar energy, and a dark reaction generating the actual "fuels" or chemicals that transport and store this energy. This second, time-lagged process is independent of the primary energy source, the sunlight, and thus ensures that fuel is continually produced over the entire diurnal cycle. This contrasts with current man-made systems, which suffer from an annoying disruption of energy production during the night.
In photovoltaic systems, solar cells generate electrons for either local use or to feed them into the public grid. Storage of electric energy is usually performed in batteries or by electrochemical conversion into fuels such as hydrogen or methane. Mimicking Nature's photosynthesis in a process known as "artificial photosynthesis" would imply using a material that is able to store the electrons right after their light-induced generation and release them on demand. Such a material was explored by Bettina V. Lotsch at the Max Planck Institute for Solid State Research, Germany, and collaborators in Zurich and Cambridge. It was obtained from "melon", an ordered carbon nitride polymer, which is currently heavily investigated for its photocatalytic and semiconducting properties.
The as-modified graphitic nitride is a yellow solid, which changes color upon exposure to light. "This polymer turns blue when photo-irradiated in the presence of certain electron donors in an oxygen-free environment," said the scientists. This "blue radical" state contains trapped electrons. The scientists found out that when the light was switched off and a hydrogen-evolution co-catalyst was added, the polymer turned yellow again while producing hydrogen by releasing the stored electrons. Thus it is possible to decouple the generation of photoinduced electrons from their use, for example, in fuel production, within one single, inexpensive material. This could be a significant advance for the production of storable solar fuels independent of the intermittency of solar irradiation.
Original publication
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.





























































