First intermetallic double salt with platinum
Scientists at the U.S. Department of Energy's Ames Laboratory are being credited with creating the first intermetallic double salt with platinum.
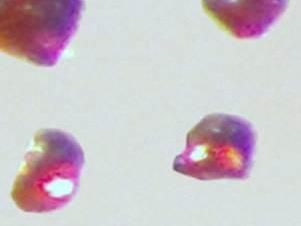
Cesium platinide hydride, or 4Cs2Pt-CsH, forms a translucent ruby red crystal and can exist only in an inert environment similar to conditions that exist in outer space
Ames Laboratory, US Department of Energy
Materials researchers Anja-Verena Mudring and Volodymyr Smetana were the first to create and accurately characterize the compound. Cesium platinide hydride, or 4Cs2Pt-CsH, forms a translucent ruby red crystal and can exist only in an inert environment similar to conditions that exist in outer space. It's a new member of a rare family of compounds in which a metal forms a truly negatively charged ion.
"It's a compound that as a researcher you have trouble envisioning that it can even exist, but once you do have it and can analyze it, it's nothing like what you expect," said Mudring. "Instead of creating a gray, shiny alloy as typically observed for many hydrogen storage materials by reacting the metals cesium and platinum with hydrogen, these red crystals form. They are really quite beautiful."
This intriguing new compound was initially extracted from a cesium melt. The compound is highly unstable, with the platinum in the compound returning to its elemental state if it is exposed to oxygen. Since first detected it took a long time to understand its true nature and prove the composition. Single crystal studies combined with powder X-ray diffraction, solid-state nuclear magnetic resonance and deep theoretical investigations allowed researchers to prove its existence. Its unusual structure and properties, so different from typical intermetallic hydrides, are explained by the strong influence of relativistic effects on both cesium and platinum.
"It's unique. It's the first example we have of a salt with so strongly negatively charged metal ions. Moreover, you mix an alloy with a salt and get another non-conducting salt," said Mudring. "This allows for some deep insight into the nature of chemical bonding--and as Goethe wrote, ultimately what holds the world and its compounds together in its inmost form."
Original publication
Volodymyr Smetana , Anja‐Verena Mudring; "Cesium Platinide Hydride 4Cs2Pt⋅CsH: An Intermetallic Double Salt Featuring Metal Anions"; Angew. Chem. Int. Ed.; 2016
Volodymyr Smetana , Anja‐Verena Mudring; "Caesiumplatinidhydrid, 4 Cs2Pt⋅CsH: ein intermetallisches Doppelsalz mit Metall-Anionen"; Angew. Chem.; 2016
Original publication
Volodymyr Smetana , Anja‐Verena Mudring; "Cesium Platinide Hydride 4Cs2Pt⋅CsH: An Intermetallic Double Salt Featuring Metal Anions"; Angew. Chem. Int. Ed.; 2016
Volodymyr Smetana , Anja‐Verena Mudring; "Caesiumplatinidhydrid, 4 Cs2Pt⋅CsH: ein intermetallisches Doppelsalz mit Metall-Anionen"; Angew. Chem.; 2016
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.



























































