Rare form of phosphorus compound discovered
Titanium phosphate displays unusual transformations at high pressure
Scientists at DESY and the European Synchrotron Radiation Facility ESRF have discovered a new and very unusual form of phosphorus compound. In high-pressure experiments, a team led by Dr. Maxim Bykov from the University of Bayreuth found evidence for a configuration where a central phosphorus atom is bound to five surrounding oxygen atoms (PO5). Such a configuration is very rare and it is the first time that PO5 groups were found in an inorganic phosphate compound.
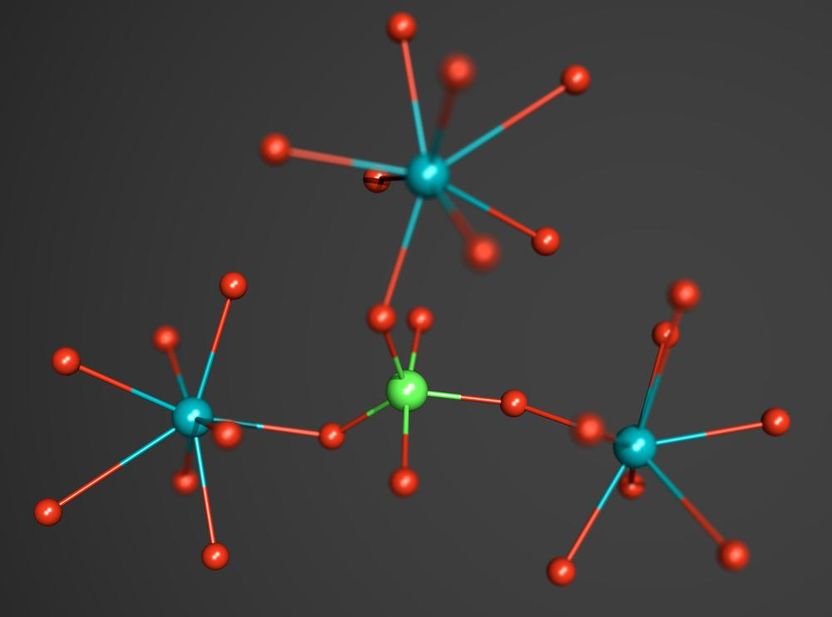
A fragment of the crystal structure of TiPO4-V. The central phosphorus atom is shown in green, titanium atoms are blue, oxygen atoms are red.
Elena Bykova, University of Bayreuth
The scientists studied titanium orthophosphate (TiPO4) at pressures of up to 56 Giga-Pascal (GPa), more than 500 000 times the average pressure at sea level. To achieve this pressure, they used a device called a diamond anvil cell, where the sample is compressed between two tiny diamond anvils. With the intense X-ray beam at DESY’s synchrotron radiation source PETRA III and using a novel methodology for single crystal diffraction experiments at high pressures they were able to distinguish and characterize four phase transitions that appeared during the compression of titanium orthophosphate to over half a million atmospheres. “In the last phase discovered at the highest achievable pressure, a central phosphorus atom was bound to – or coordinated by – five surrounding oxygen atoms,” explains Bykov.
Phosphorus plays a key role in the chemistry of life and many technological applications. Unusually high coordination numbers of cations like phosphorus are often associated with interesting physical properties like high density, ultra-high hardness or low compressibility, which are very relevant for engineering applications. Pentacoordinated (5-coordinated) phosphorus is expected to be especially chemically active and thus may play a significant role in the synthesis of novel organic or metal-organic compounds. The experimental demonstration that phosphorus could form PO5 polyhedra suggests that similar compounds may appear as intermedia (metastable, short-time leaving, and hardly or not at all detectable) products of different chemical processes involving phosphorus and oxygen. “The creation of new compounds based on PO5 groups could have significant implications for industrial applications if someone finds a way to recover these materials at ambient conditions and study their physical properties,” says Bykov.
When they started their experiment, Bykov and his colleagues were actually looking to examine another phenomenon: titanium orthophosphate shows very unusual magnetic properties. Under normal pressure it forms a so called quasi-one-dimensional antiferromagnetic system. When it is cooled down, TiPO4 loses this form of magnetism and transforms to a diamagnetic state. This process is called spin-Peierls transition. The scientists were now able to prove that this transition also happens when TiPO4 is put under high pressure (above 7 GPa). “The result has important consequences for studying this process in more detail. It both improves our understanding of the spin-Peierls transitions and reveals new possibilities to examine this transition without low temperatures” explains Bykov.
Original publication
Most read news
Original publication
Maxim Bykov, Elena Bykova, Michael Hanfland, Hanns-Peter Liermann, Reinhard K. Kremer, Robert Glaum, Leonid Dubrovinsky, Sander van Smaalen; "High-Pressure Phase Transformations in TiPO4: A Route to Pentacoordinated Phosphorus"; Angewandte Chemie; 2016
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.