Researchers use graphene templates to make new metal-oxide nanostructures
Researchers from Brown University have found a new method for making ultrathin metal-oxide sheets containing intricate wrinkle and crumple patterns. In a study the researchers show that the textured metal-oxide films have better performance when used as photocatalysts and as battery electrodes.
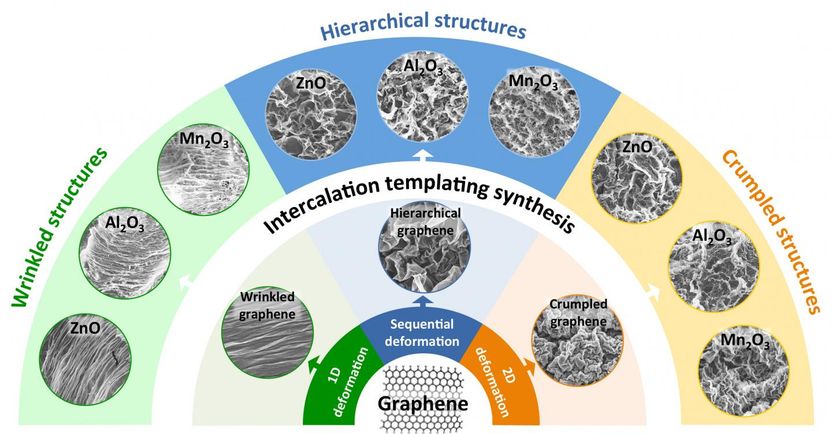
Researchers from Brown University have developed a method of using graphene templates to make metal-oxide films with intricate surface textures. A study shows that those textures can enhance the performance of the films as battery electrodes and as photocatalysts.
Hurt lab / Wong lab / Brown University
The new findings build on previous work done by the same research group in which they developed a method for introducing finely tuned wrinkle and crumple textures into sheets of the nanomaterial graphene oxide. The study showed that the process enhanced some of graphene's properties. The textures made the graphene better able to repel water, which would be useful in making water-resistant coatings, and enhanced graphene's ability to conduct electricity.
The researchers thought that similar structures might enhance the properties of other materials -- specifically metal oxides -- but there's a problem. To introduce wrinkle and crumple structures in graphene, the team compressed the sheets multiple times in multiple orientations. That process won't work for metal oxides.
"Metal oxides are too stiff," said Po-Yen Chen, a Hibbitt Postdoctoral Researcher in Brown's School of Engineering who led the work. "If you try to compress them, they crack."
So Chen, working with the labs of Robert Hurt and Ian Y. Wong, both engineering professors at Brown, developed a method of using the crumpled graphene sheets as templates for making crumpled metal-oxide films.
"We showed that we can transfer those surface features from the graphene onto the metal oxides," Chen said.
The team started by making stacks of crumpled graphene sheets using the method they had developed previously. They deposited the graphene on a polymer substrate that shrinks when heated. As the substrate shrinks, it compresses the graphene sitting on top, creating wrinkle or crumple structures. The substrate is then removed, leaving free-standing sheets of crumpled graphene behind. The compression process can be done multiple times, creating ever more complex structures. The process also allows control of what types of textures are formed. Clamping shrink film on opposite sides and shrinking it in only one direction creates periodic wrinkles. Shrinking in all directions creates crumples. These shrinks can be performed multiple times in multiple configurations to create a wide variety of textures.
To transfer those patterns onto metal oxides, Chen placed the stacks of wrinkled graphene sheets in a water-based solution containing positively charged metal ions. The negatively charged graphene pulled those ions into the spaces between the sheets. The particles bonded together within the interlayer space, creating thin sheets of metal that followed the wrinkle patterns of the graphene. The graphene was then oxidized away, leaving the wrinkled metal-oxide sheets. Chen showed that the process works with a variety of metal oxides -- zinc, aluminum, manganese and copper oxides.
Once they had made the materials, the researchers then tested them to see if, as was the case with graphene, the textured surfaces enhanced the metal oxides' properties.
They showed that wrinkled manganese oxide, when used as a battery electrode, had charge-carrying capacity that was four times higher than a planar sheet. That's probably because the wrinkle ridges give electrons a defined path to follow, enabling the material to carry more of them at a time, the researchers say.
The team also tested the ability of crumpled zinc oxide to perform a photocatalytic reaction -- reducing a dye dissolved in water under ultraviolet light. The experiment showed the crumpled zinc oxide film to be four times more reactive than a planar film. That's probably because the crumpled films have higher surface area, which give the material more reactive sites, Chen said.
In addition to improving the properties of the metals, Chen points out that the process also represents a way of making thin films out of materials that don't normally lend themselves to ultrathin configurations.
"Using graphene confinement, we can guide the assembly and synthesis of materials in two dimensions," he said. "Based on what we learned from making the metal-oxide films, we can start to think about using this method to make new 2D materials that are otherwise unstable in bulk solution. But with our confinement method, we think it's possible."