Ionic liquids simplify laser experiments on liquid samples
An HZB team has developed a new approach to conduct photoemission spectroscopy of molecules in solution. This has been difficult up to now because the sample needed to be situated in vacuum – but liquids evaporate. The work has now demonstrated it is feasible to replace the solvent with an ionic liquid of low vapor pressure, which does not perturb the sample characteristics. This allows the molecules to be excited with a laser pulse and to record the behaviour of the excited energy states. It provides insight into the physical and chemical processes of novel liquid energy materials that might be employed in organic solar cells or catalysts, for instance.
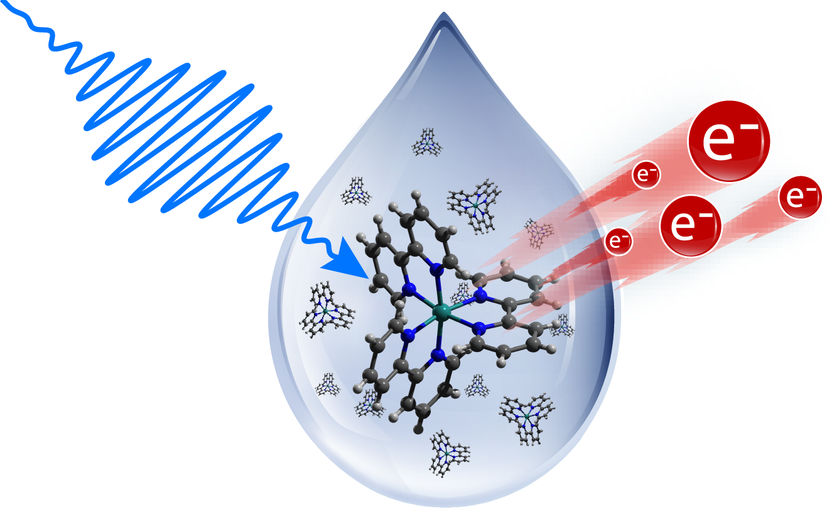
A pump pulse promotes the molecule's electrons to an excited state and with probe pulses the binding energy of the excited electrons can be measured. However, pump-probe laser experiments are only feasible under ultra-high vacuum.
HZB
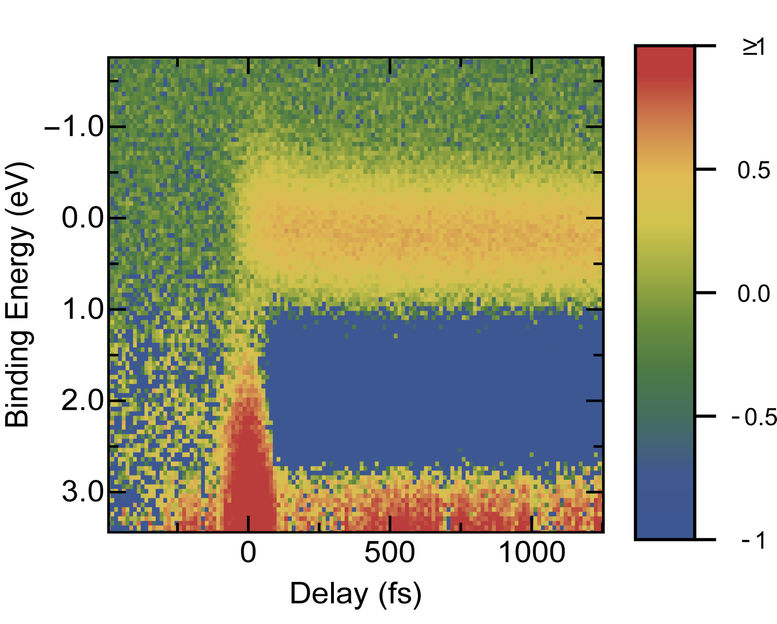
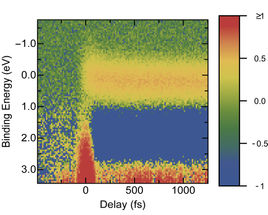
This graph shows the relative amount of electrons (coloured scale, right) at a given binding energy shortly after the pump pulse. This information helps to reconstruct how electrons relax from their excited states to the ground state.
HZB
Numerous processes take place in solution not only in nature, but in engineering applications as well. One example is organic solar cells made of dissolved dye molecules; another is a new class of catalyst materials consisting of nanoparticles in solution. Time-resolved photoelectron spectroscopy (PES) is well suited to reveal the processes triggered by light in these systems of materials. A precisely tuned excitation pulse from a laser, called the pump pulse, promotes electrons in the sample to an excited state, whereas application of a precisely timed and short probe pulse allows to measure the binding energy of the excited electrons. This permits reconstruction of how the excited electrons fall back to their ground state, and conclusions to be drawn about the physical, chemical, and biological processes that can take place in these materials. However, pump-probe laser experiments detecting photoelectrons are only feasible under ultra-high vacuum. The method is well-established for solid-state matter, but not for liquid samples. Liquids immediately evaporate in vacuum. Up to now, they could only be studied with costly techniques and with the use of equipment like the liquid micro jet.
Ionic liquids never evaporate
Now a group headed by Prof. Emad Aziz has shown for the first time that there is a simple alternative for conducting time-resolved PES experiments on dissolved samples. They replace the solvent with what is called an ionic liquid. This is made up of organic molecules networked to one another through ionic forces (i.e. like a salt), which compose a liquid at room temperature. Ionic liquids do not evaporate even under ultra-high vacuum.
Red dye tested
The team was successful in dissolving a prototype red dye for organic solar cells in an ionic liquid and studying it with photoelectron spectroscopy. This involved exciting the dye with a laser pulse. During the first picosecond (10-12s) afterwards, the probe pulses sampled the binding energy of the excited electrons 150 times. The graph produced from the data shows the intermediate energy states at which the excited electrons emit their energy. Since light-induced processes for this dye have already been studied in depth, the physicists were able to compare their experimental data with existing results.
Perfect results with this new method
“The alternative solvent has no influence on the ultrafast processes. All processes that take place during the first picosecond correspond perfectly to results of measurements in conventional solvents as well as to simulations”, explains Mario Borgwardt, who carried out the experiments as part of his doctoral dissertation. An important result: it is the fast processes that lead to losses in a solar cell, for example. Summing up, Emad Aziz has no doubt that “ionic liquids are a good alternative to conventional solvents for analysing molecules in solution using time-resolved photoelectron spectroscopy.”
Outlook: exploring light reactive catalysts
Now the team intends to investigate nanoparticles in ionic liquids using PES, in particular nanodiamonds made of carbon. In DIACAT, a large cooperative project at HZB involving many partnering organisations, scientists are testing the suitability of nanodiamonds as light-sensitive catalysts for producing solar fuels. The new methodology has come at just the right time.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!