One-pot synthesis towards sulfur-based organic semiconductors
A short and simple synthetic route for thiophene-fused aromatic compounds
Dr. Lingkui Meng, Dr. Yasutomo Segawa, Professor Kenichiro Itami of the JST-ERATO Itami Molecular Nanocarbon Project, Institute of Transformative Bio-molecules (ITbM) of Nagoya University and Integrated Research Consortium on Chemical Sciences, and their colleagues have reported on the development of a simple and effective method for the synthesis of thiophene-fused PAHs.
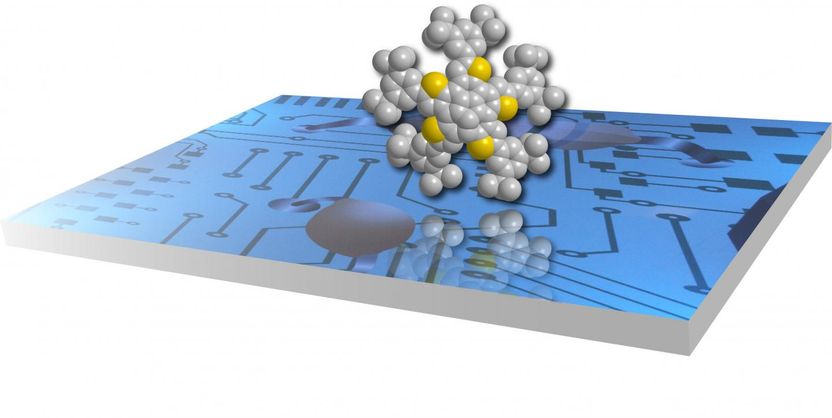
Yellow and gray colors on the molecule represent sulfur and carbon atoms respectively. Thiophene-fused PAHs have found uses as transistors.
ITbM, Nagoya University
Thiophene-fused PAHs are organic molecules composed of multiple aromatic rings including thiophene.
ITbM, Nagoya University
Thiophene-fused PAHs are organic molecules composed of multiple aromatic rings including thiophene. Thiophene is a five-membered aromatic ring containing four carbon atoms and a sulfur atom. Thiophene-fused PAHs are known to be one of the most common organic semiconductors and are used in various electronic materials, such as in transistors, organic thin-film solar cells, organic electro-luminescent diodes and electronic devices. More recently, they have found use in wearable devices due to their lightweight and flexibility.
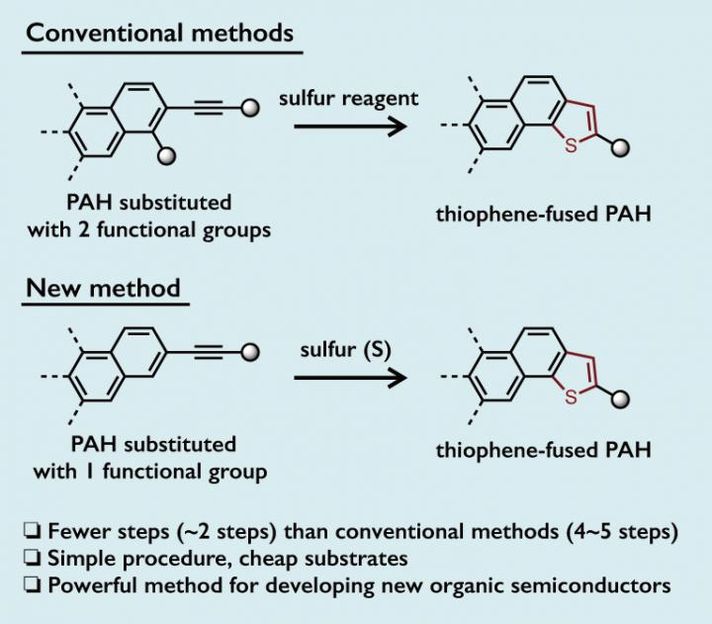
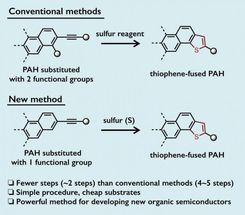
Thienannulation (thiophene-annulation) reactions, a transformation that makes new thiophene rings via cyclization, leads to various thiophene-fused PAHs. Most conventional thienannulation methods require the introduction of two functional groups adjacent to each other to form two reactive sites on PAHs before the cyclization can take place. Thus, multiple steps are required for the preparation of the substrates. As a consequence, a more simple method to access thiophene-fused PAHs is desirable.
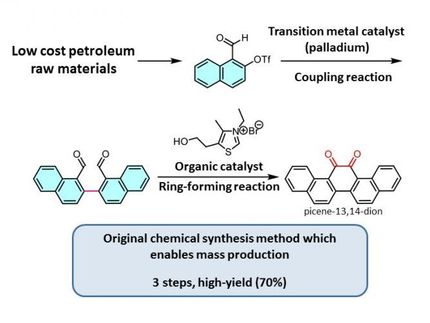
A team led by Yasutomo Segawa, a group leader of the JST-ERATO project, and Kenichiro Itami, the director of the JST-ERATO project and the center director of ITbM, has succeeded in developing a simple and effective method for the formation of various thiophene-fused PAHs. They have managed to start from PAHs that have only one functional group, which saves the effort of installing another functional group, and have performed the thienannulation reactions using elemental sulfur, a readily available low cost reagent. The reactions can be carried out on a multigram scale and can be conducted in a one-pot two-step reaction sequence starting from an unfunctionalized PAH. This new approach can also generate multiple thiophene moieties in a single reaction. Hence, this method has the advantage of offering a significant reduction in the number of required steps and in the reagent costs for thiophene-fused PAH synthesis compared to conventional methods.
The researchers have shown that upon heating and stirring the dimethylformamide solution of arylethynyl group-substituted PAHs and elemental sulfur in air, they were able to obtain the corresponding thiophene-fused PAHs. The arylethynyl group consists of an alkyne (a moiety with a carbon-carbon triple bond) bonded to an aromatic ring. The reaction proceeds via a carbon-hydrogen (C-H) bond cleavage at the position next to the arylethynyl group (called the ortho-position) on PAHs, in the presence of sulfur. As the ortho-C-H bond on the PAH can be cleaved under the reaction conditions, prior functionalization (installation of a functional group) becomes unnecessary.
Arylethynyl-substituted PAHs are readily accessible by the Sonogashira coupling, which is a cross-coupling reaction to form carbon-carbon bonds between an alkyne and a halogen-substituted aromatic compound. The synthesis of thiophene-fused PAHs can also be carried out in one-pot, in which PAHs are subjected to a Sonogashira coupling to form arylethynyl-substituted PAHs, followed by direct treatment of the alkyne with elemental sulfur to induce thienannulation.
"Actually, we coincidentally discovered this reaction when we were testing different chemical reactions to synthesize a new molecule for the Itami ERATO project," says Yasutomo Segawa, one of the leaders of this study. "At first, most members including myself felt that the reaction may have already been reported because it is indeed a very simple reaction. Therefore, the most difficult part of this research was to clarify the novelty of this reaction. We put in a significant amount of effort to investigate previous reports, including textbooks from more than 50 years ago as well as various Internet sources, to make sure that our reaction conditions had not been disclosed before," he continues.
The team succeeded in synthesizing more than 20 thiophene-fused PAHs. They also revealed that multiple formations of thiophene rings of PAHs substituted with multiple arylethynyl groups could be carried out all at once. Multiple thiophene-fused PAHs were generated from three-fold and five-fold thienannulations, which generated triple thia[5]helicene (containing three thiophenes) and pentathienocorannulene (containing five thiophenes), respectively. The pentathienocorannulene was an unprecedented molecule that was synthesized for the first time.
"I was extremely happy when I was able to obtain the propeller-shaped triple thia[5]helicene and hat-shaped pentathienocorannulene, because I have always been aiming to synthesize exciting new molecules since I joined Professor Itami's group," says Lingkui Meng, a postdoctoral researcher who mainly conducted the experiments. "We had some problems in purifying the compounds but we were delighted when we obtained the crystal structures of the thiophene compounds, which proved that the desired reactions had taken place."
"The best part of this research for me is to discover that our C-H functionalization strategy on PAHs could be applied to synthesize structurally beautiful molecules with high functionalities," says Segawa. "The successful synthesis of a known high-performance organic semiconductive molecule, (2,6-bis(4-n-octylphenyl)- dithieno[3,2-b:2?,3?-d]thiophene, from a relatively cheap substrate opens doors to access useful thiophene compounds in a rapid and cost-effective manner."
"We hope that ongoing advances in our method may lead to the development of new organic electronic devices, including semiconductor and luminescent materials," say Segawa and Itami. "We are considering the possibilities to make this reaction applicable for making useful thiophene-fused PAHs, which would lead to the rapid discovery and optimization of key molecules that would advance the field of materials science."
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.