Jean-Pierre Sauvage, Fraser Stoddart und Bernard Feringa awarded with the Nobel Prize
Noble Prize for Chemistry 2016
Advertisement
A tiny lift, artificial muscles and miniscule motors. The Nobel prize in Chemistry 2016 is awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart and Bernard L. Feringa for their design and production of molecular machines. They have developed molecules with controllable movements, which can perform a task when energy is added.
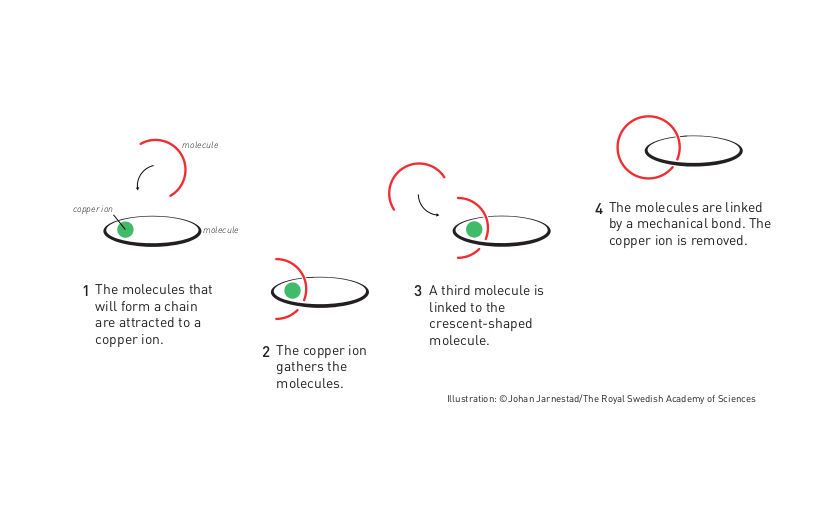
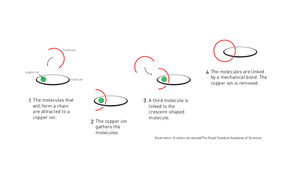
Molecular chain
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
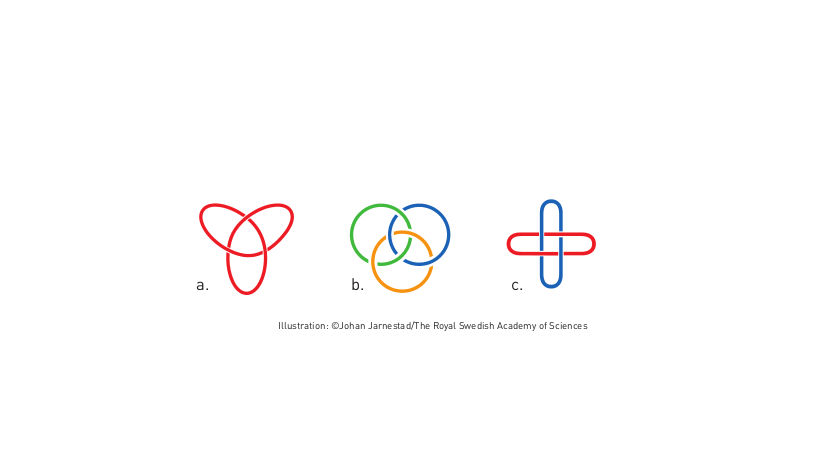
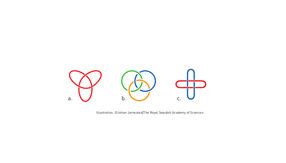
Molecular knots
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
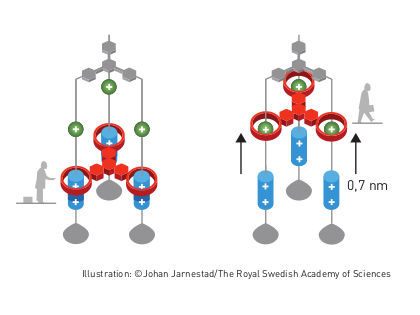
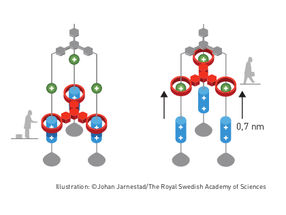
Molecular elevator
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
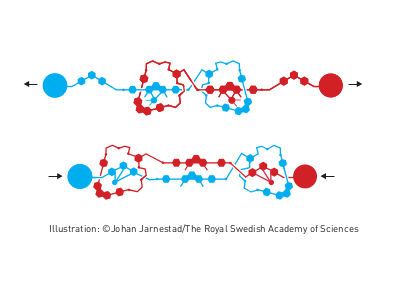
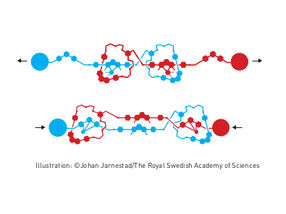
Elastic molecular structure - Molecular muscle
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
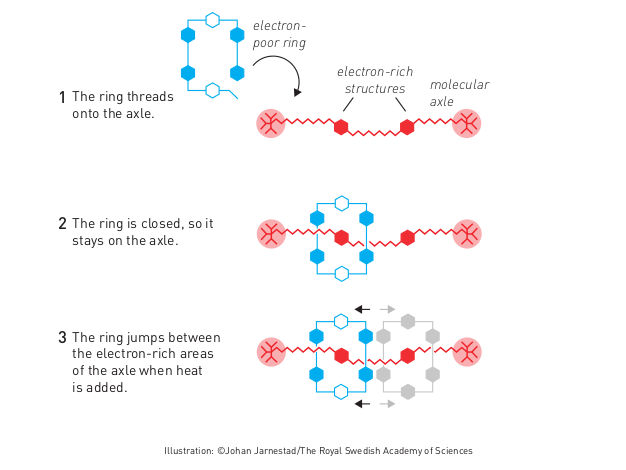
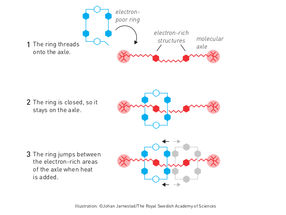
Molecular shuttle
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
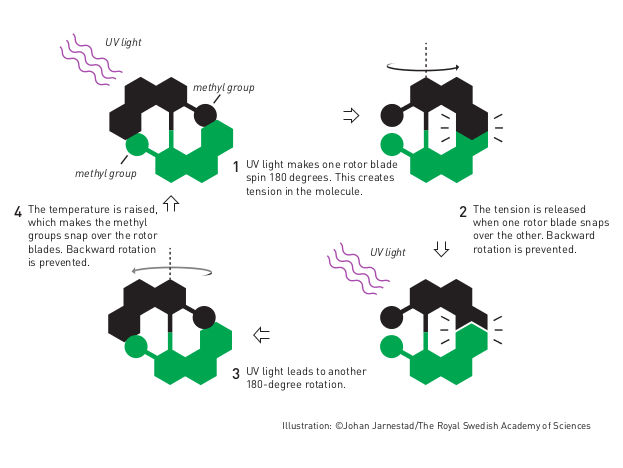
Molecular motor
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
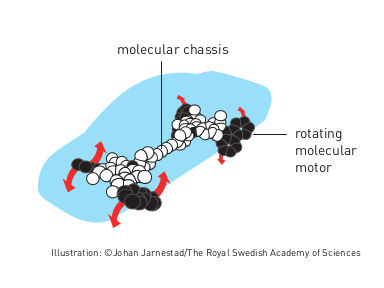
Nanocar
Illustration: ©Johan Jarnestad/The Royal Swedish Academy of Sciences
They developed the world's smallest machines
The development of computing demonstrates how the miniaturisation of technology can lead to a revolution. The 2016 Nobel Laureates in Chemistry have miniaturised machines and taken chemistry to a new dimension.
The first step towards a molecular machine was taken by Jean-Pierre Sauvage in 1983, when he succeeded in linking two ring-shaped molecules together to form a chain, called a catenane. Normally, molecules are joined by strong covalent bonds in which the atoms share electrons, but in the chain they were instead linked by a freer mechanical bond. For a machine to be able to perform a task it must consist of parts that can move relative to each other. The two interlocked rings fulfilled exactly this requirement.
The second step was taken by Fraser Stoddart in 1991, when he developed a rotaxane. He threaded a molecular ring onto a thin molecular axle and demonstrated that the ring was able to move along the axle. Among his developments based on rotaxanes are a molecular lift, a molecular muscle and a molecule-based computer chip.
Bernard Feringa was the first person to develop a molecular motor; in 1999 he got a molecular rotor blade to spin continually in the same direction. Using molecular motors, he has rotated a glass cylinder that is 10,000 times bigger than the motor and also designed a nanocar.
2016's Nobel Laureates in Chemistry have taken molecular systems out of equilibrium's stalemate and into energy-filled states in which their movements can be controlled. In terms of development, the molecular motor is at the same stage as the electric motor was in the 1830s, when scientists displayed various spinning cranks and wheels, unaware that they would lead to electric trains, washing machines, fans and food processors. Molecular machines will most likely be used in the development of things such as new materials, sensors and energy storage systems.
The laureates
- Jean-Pierre Sauvage, born 1944 in Paris, France. Ph.D. 1971 from the University of Strasbourg, France. Professor Emeritus at the University of Strasbourg and Director of Research Emeritus at the National Center for Scientific Research (CNRS), France.
- Sir J. Fraser Stoddart, born 1942 in Edinburgh, UK. Ph.D. 1966 from Edinburgh University, UK. Board of Trustees Professor of Chemistry at Northwestern University, Evanston, IL, USA.
- Bernard L. Feringa, born 1951 in Barger-Compascuum, the Netherlands. Ph.D.1978 from the University of Groningen, the Netherlands. Professor in Organic Chemistry at the University of Groningen, the Netherlands.