Researchers find thin layers of water can become ice-like at room temperature
New research by scientists at The University of Akron (UA) shows that a nanometer-thin layer of water between two charged surfaces exhibits ice-like tendencies that allow it to withstand pressures of hundreds of atmospheres. The discovery could lead to better ways to minimize friction in a variety of settings.
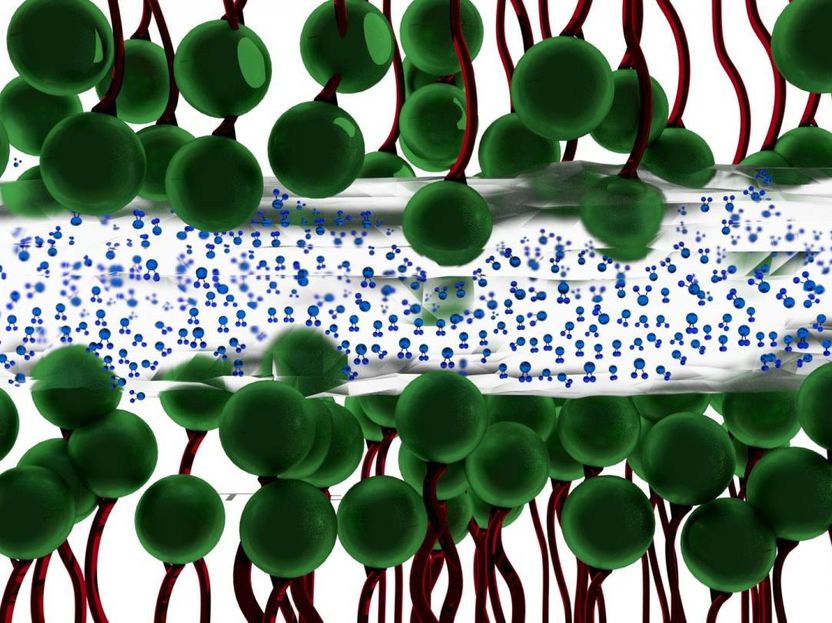
Researchers at The University of Akron have discovered that a thin layer of water (blue molecules ) between two charged surfaces composed of surfactants (green molecules) --becomes ice-like, lessening the friction between the two surfaces.
The University of Akron
Why water between two surfaces does not always simply squeeze out when placed under severe pressure had never been fully understood. The UA researchers discovered that naturally-occurring charges between two surfaces under intense pressure traps the water, and gives it ice-like qualities. It is this ice-like layer of water--occurring at room temperature--that then lessens the friction between the two surfaces.
"For the first time we have a basic understanding of what happens to water under these conditions and why it keeps two surfaces apart," says Professor Ali Dhinojwala. "We had suspected something was happening at the molecular level, and now we have proof."
"This discovery could lead to improved designs where low friction surfaces are critically important, such as in biomedical knee implants," says UA graduate student Nishad Dhopatkar.
Graduate student Adrian Defante, who was also part of the research team, says "the newfound properties of water might contribute to the development of more effective antimicrobial coatings, as a thin layer of water could prevent bacterial adhesion."
Dhinojwala adds that the research conversely offers insight into how water might be kept away from two surfaces, which could lead to better adhesives in watery environments.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Luis_E._Miramontes
Haplogroup_E_(Y-DNA)

New process for natural-based microparticles in cosmetics - Research against microplastics
Speeding up extreme fast charging capability in lithium-ion batteries - “Extreme” fast charging, high energy density, and cycle life are the “holy grail” of features that the automobile industry seeks out in batteries

Pioneering plasma-catalytic process for CO2 hydrogenation to methanol under ambient conditions





























































