Lonely Atoms, Happily Reunited
The remarkable behaviour of platinum atoms on magnetite surfaces could lead to better catalysts. Scientists at TU Wien (Vienna) can now explain how platinum atoms can form pairs with the help of carbon monoxide.
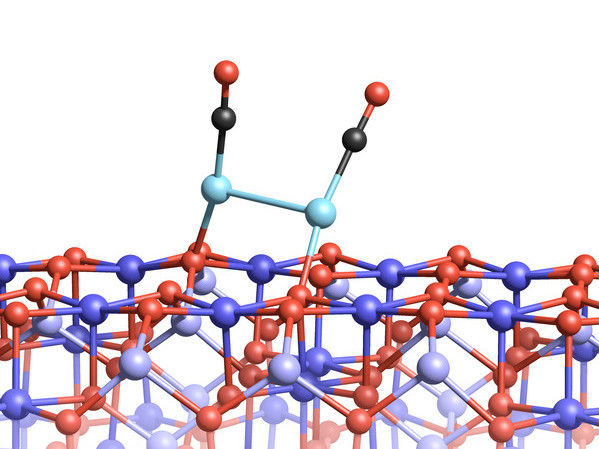
Two platinum atoms on the magnetite surface can bond, if they are attached to CO molecules.
TU Wien
At first glance, magnetite appears to be a rather inconspicuous grey mineral. But on an atomic scale, it has remarkable properties: on magnetite, single metal atoms are held in place, or they can be made to move across the surface. Sometimes several metal atoms on magnetite form small clusters. Such phenomena can dramatically change the chemical activity of the material. Atomic processes on the magnetite surface determine how well certain metal atoms can serve as catalysts for chemical reactions.
Scientists at TU Wien (Vienna), together with colleagues from Utrecht University, can now watch single platinum atoms form tiny clusters. Carbon monoxide plays a dual role in this process: It allows single platinum atoms to move and form pairs, and then it holds these pairs together for a long time. Only by increasing the temperature can the pair-bonds between platinum atoms can be broken.
Lonely Atoms
It sounds a bit like an unhappy love story: "Two platinum atoms would actually like to be together, but the magnetite surface keeps them apart", says Roland Bliem (TU Wien). Together with Professor Gareth Parkinson, Professor Ulrike Diebold and their colleagues, he analysed the behaviour of platinum atoms using a scanning tunnelling microscope.
"When a platinum atom hits the magnetite surface, it is kept in place by the oxygen atoms in the magnetite. The atoms always end up alone. On other surfaces, pair formation would be favoured, but magnetite does not allow that", says Roland Bliem. The platinum atoms sit on specific places on the magnetite crystal and cannot get away without outside help.
However, with the appearance of carbon monoxide, the situation changes completely: "A carbon monoxide molecule can attach to a platinum atom and lift it up", says Gareth Parkinson. "We call that the skyhook effect." The lifting process frees the atom from the tight grip of the magnetite, and together, the molecule and the platinum atom can start moving around randomly across the magnetite surface.
When one mobilized platinum atom finds another, they can form a bond – as long as both of them are being lifted up by carbon monoxide, diminishing the influence of the magnetite below.
When the temperature is increased to 250°C, the carbon monoxide separates from the platinum atom and the bond breaks up. The two platinum atoms must once again find separate places on the magnetite surface. This effect opens up a strategy to turn clusters into single atoms – an important process in so called "single-atom catalysts". Sometimes clusters of several atoms are formed. These larger clusters, however, cannot be broken up, even at high temperatures.
Movies with Atomic Resolution
"In our scanning tunnelling microscope, we can image the same part of the surface again and again, so that we can create a movie, showing the dancing atoms", says Roland Bliem. "This is crucial for understanding what really happens on the magnetite surface. We can watch single atoms as they wander across the magnetite surface or bond with each other. If we only had a picture of the end result, we could not say with certainty, whether one specific structure consists of one, two or more atoms. Only by following the time evolution of the atomic motion, we know which interpretation is correct." Bliem did not only conduct the experiments, he also performed complex theoretical calculations to explain the peculiar behaviour of the platinum atoms on a quantum mechanical level.
For chemical catalysis, such findings play an important role. "Metals such as platinum are frequently used as catalysts”, says Gareth Parkinson. “But a large cluster of many metal atoms may have completely different chemical properties than single metal atoms sitting separately on a surface. When we want to optimize catalysts, so we must be able to understand and control the behaviour of the atoms. This work is one step further towards that goal."
Original publication
Most read news
Original publication
Roland Bliema, Jessi E. S. van der Hoevenb, Jan Hulvaa, Jiri Paveleca, Oscar Gambaa, Petra E. de Jonghb, Michael Schmida, Peter Blahac, Ulrike Diebolda, and Gareth S. Parkinson; "Dual role of CO in the stability of subnano Pt clusters at the Fe3O4(001) surface". PNAS; 2016
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Advanced imaging for bone research and materials science - High-resolution method for computed nano-tomography developed
MSC unanimously agrees that Bisphenol A is an endocrine disruptor
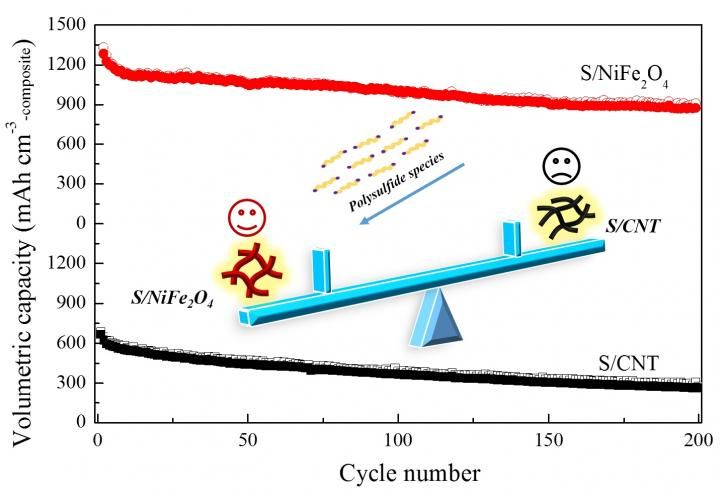
Nickel ferrite promotes capacity and cycle stability of lithium-sulfur battery

Clues to accelerate commercialization of all-solid-state batteries - New findings reveal how degradation of all-solid-state batteries occurs at the cathode under low-pressure operation
Second European refinery to run Headwaters heavy oil catalyst technology

Nacelle: Launch of the first independent sodium-ion production line in Germany - Milestone for German battery technology

New battery technology could boost renewable energy storage - Columbia Engineers develop new powerful battery "fuel" -- an electrolyte that not only lasts longer but is also cheaper to produce