Rechargeable batteries that last longer and re-charge more rapidly
Advertisement
Materials researchers at the Swiss Paul Scherrer Institute PSI in Villigen and the ETH Zurich have developed a very simple and cost-effective procedure for significantly enhancing the performance of conventional Li-ion rechargeable batteries. The procedure is scalable in size, so the use of rechargeable batteries will be optimized in all areas of application—whether in wristwatches, smartphones, laptops or cars. Battery storage capacity will be significantly extended, and charging times reduced.
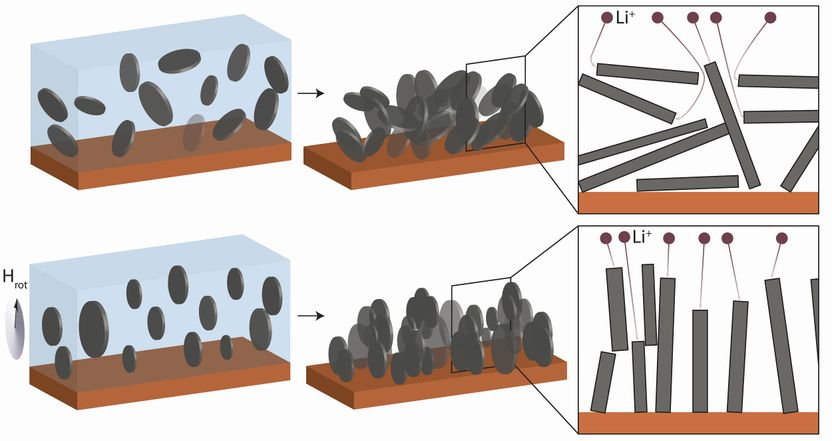
Haphazardly arranged graphite flakes in a conventional anode (above left and center): lithium ions attempting to dock or return to the cathode are forced to take detours (above right). But if the graphite is subjected to a rotating magnetic field (below), the flakes in the suspension align themselves vertically in parallel formation. They keep this orientation after they have been dried (below centre). The ions have shorter paths (below right).
Graphics: Juliette Billaud, Florian Bouville, Tommaso Magrini/Paul Scherrer Institute, ETH Zurich
It’s not necessary to re-invent the rechargeable battery in order to improve its performance. As Claire Villevieille, head of the battery materials research group at the Paul Scherrer Institute PSI says: In the context of this competitive field, most researchers concentrate on the development of new materials.
In cooperation with colleagues at the ETH in Zurich, Villevieille and co-researcher Juliette Billaud took a different approach: We checked existing components with a view to fully exploiting their potential.
Simply by optimizing the graphite anode – or negative electrode - on a conventional Li-ion battery, researchers were able to boost battery performance. Under laboratory conditions, we were able to enhance storage capacity by a factor of up to 3. Owing to their complex construction, commercial batteries will not be able to fully replicate these results. But performance will definitely be enhanced, perhaps by as much as 30 – 50 percent: further experiments should yield more accurate prognoses.
Researchers point out that in terms of industrial implementation, improving existing components has the great advantage of requiring less developmental input than a new battery design using new materials. As Villevieille says: We already have everything we need. If a manufacturer were willing to take on production, enhanced batteries could be ready for the market within one or two years.
The procedure is simple, cost-effective and scalable for use on rechargeable batteries in all areas of application, from wristwatch to smartphone, from laptop to car. And it has the additional bonus of being transferable to other anode-cathode batteries such as those based on sodium.
Arranging the flakes
In this case, changing the way anodes work was the key to success. Anodes are made from graphite, i.e. carbon, arranged in tiny, densely packed flakes, comparable in appearance to dark grey cornflakes haphazardly compressed, as in a granola bar. When a Li-ion battery is charging, lithium ions pass from the cathode, or positive metal oxide electrode, through an electrolyte fluid to the anode, where they are stored in the graphite bar. When the battery is in use and thus discharging, the lithium ions pass back to the cathode but are forced to take many detours through the densely packed mass of graphite flakes, compromising battery performance.
These detours are largely avoidable if the flakes are arranged vertically during the anode production process so that they are massed parallel to one another, pointing from the electrode plane in the direction of the cathode. Adapting a method already used in the production of synthetic composite materials, this alignment was achieved by André Studart and a team of research experts in the field of material nanostructuration at the ETH Zurich. The method involves coating the graphite flakes with nanoparticles of iron oxide sensitive to a magnetic field and suspending them in ethanol. The suspended and already magnetized flakes are subsequently subjected to a magnetic field of 100 millitesla—about the strength of a fridge magnet. André Studart explains that by rotating the magnet during this process, the platelets not only align vertically but in parallel formation to one another, like books on a shelf. As a result, they are perfectly ordered, reducing the diffusion distances covered by the lithium ions to a minimum.
Shorter paths for the ions
Microscopic images show that if the magnet remains turned on during the ensuing drying process, the platelets keep their new orientation even when removed from the ethanol suspension. Instead of their formerly haphazard arrangement, the flakes in the compressed graphite bar are now parallel, enabling the lithium ions to flow much more easily and quickly, whilst also increasing storage capacity by allowing more ions to dock during the charging process. Claire Villevieille emphasizes that the chemical composition of batteries remains the same
. The remaining iron oxide nanoparticles are negligible in quantity and do not influence battery function. All we did was optimise the anode structure.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.