A new way to control oxygen for electronic properties
Hotel managers and materials scientists have a lot in common--they both need to find a way to control properties by managing vacancies.

Argonne physicist Jeff Eastman holds a thin oxide film which dramatically increases its conductivity when a small current is applied. The discovery could be useful in studies for better electronics or catalysts.
Photo by Mark Lopez/Argonne National Laboratory.
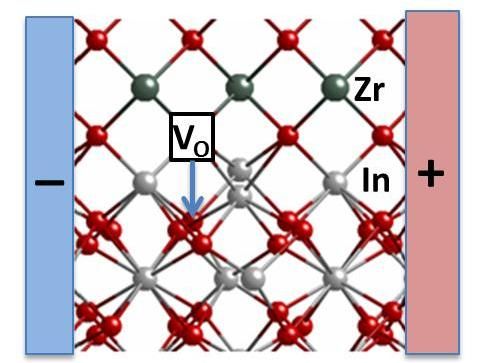
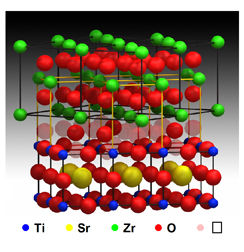
This picture shows a new system designed by Argonne researchers consisting of indium oxide and yttria-stabilized zirconia layers. They found that when they applied a small electric field in a horizontal direction, between the "+" and "-" regions, some oxygen vacancies move vertically from the yttria-stabilized zirconia into the indium oxide. This causes its electrical conductivity to increase substantially and could be useful in electronics or catalyst applications. Zirconium ions are shown in green, oxygen ions are red, and indium ions are silver.
Image courtesy Jeff Eastman/Nature.

Researchers at the U.S. Department of Energy's Argonne National Laboratory found they could use a small electric current to introduce oxygen voids, or vacancies, that dramatically change the conductivity of thin oxide films.
The discovery improves our understanding of how these materials work and could be useful for new electronics, catalysts or more.
Scientists are always looking for unusual behaviors in materials that could form the basis of new technologies. Oxides are a class of materials that has garnered much recent interest because they sometimes display such unusual behaviors--flipping between insulating and conducting states, turning magnetism on and off, or even becoming superconducting: conducting electricity perfectly, without any loss as heat.
We think some of these properties have to do with oxygen vacancies. The structure of an oxide is a repeating crystalline lattice with oxygen atoms peppered throughout; but sometimes there may be voids where an oxygen atom is missing.
The usual way to create oxygen vacancies is by heating the materials and adding or removing oxygen from the environment.
"But the need to control the gas environment limits where and when you can change the material's properties," said Jeff Eastman, an Argonne materials scientist and corresponding author on the paper.
The Argonne team wanted to find out if they could control vacancies with an alternate method.
They built a two-layer material: an indium oxide crystal layer on top of a block of yttria-stabilized zirconia. When the researchers applied a small electric field, they watched the electrical conductivity skyrocket by two orders of magnitude along the boundary where the two layers meet. The effect is reversible; without the field, it reverts back to the initial, less conductive state.
"You could imagine applications for electronics or building catalysts--for example, providing a way to split water or carbon dioxide," Eastman said.
The theory, assisted by computational modeling, is that the difference between the two materials' properties creates a vertical voltage between them, and negatively charged oxygen ions in the indium oxide are attracted to the flow and move across the interface--leaving vacancies behind.
The team is planning further investigation into whether the same effects occur in other materials and whether the method could control other properties, Eastman said.