Surface wetting – tracking down the causes of polar hydrophobicity
The question of whether a liquid beads or adheres to a surface plays a role in almost all branches of industry. Researchers from the Fraunhofer Institute for Mechanics of Materials IWM in Freiburg and ExxonMobil Research & Engineering in New Jersey have now developed a multiscale simulation method for predicting the wetting behavior of liquids on surfaces. In a recent edition of the Journal of the American Chemical Society, the research team applied this methodology to the previously unexplained phenomenon of polar hydrophobicity in fluorinated carbon surfaces.
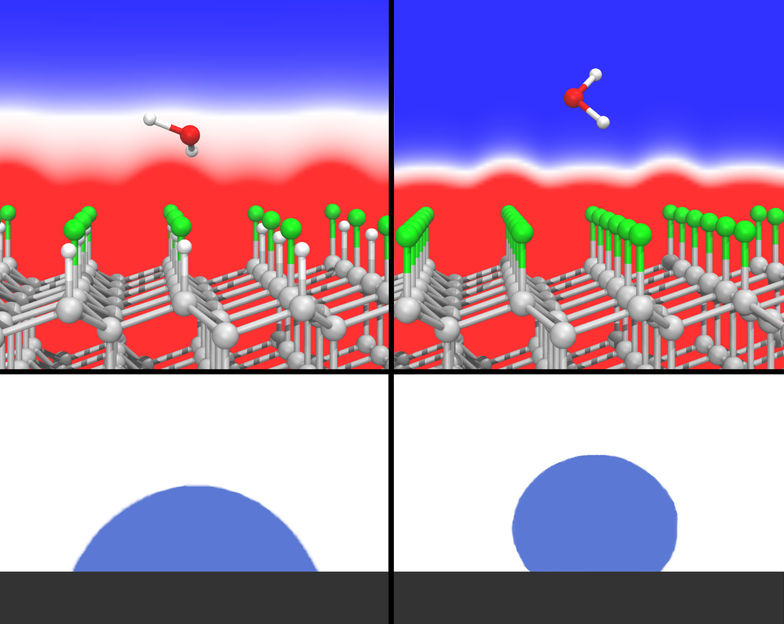
On the diamond surface (left) an adsorbed water molecule interacts with a strong electric field, at the fully fluorinated surface however, the water molecule adsorbs in a practically field free zone.
Fraunhofer Institute for Mechanics of Materials IWM
The research team, comprising Dr. Leonhard Mayrhofer, Dr. Gianpietro Moras, Dr. Narasimham Mulakaluri, and group manager Prof. Michael Moseler from the Fraunhofer IWM, MikroTribologie Centrum µTC, as well as Dr. Srinivasan Rajagopalan and Dr. Paul A. Stevens from Corporate Strategic Research, ExxonMobil Research & Engineering, can point to success at several levels. “For one thing, the behavior of liquids on surfaces can now be predicted by means of a quantum-mechanical description of the valence electrons,” says Mayrhofer, first author. For another, the researchers believe they can use their work to now close a gap in the understanding of polar hydrophobicity, as it is called, for fluorinated carbon surfaces – that had long remained an open question. This effect had already been observed when Roy Plunkett discovered Teflon® in 1938. Teflon, like nearly all perfluorinated carbon materials, is remarkably water-repellent, i.e. hydrophobic. Although the carbon-fluorine bonding exhibits a high degree of polarity, water molecules of similarly strong polarity surprisingly do not bind well to the surface. The research team has now been able for the first time to explain the origin of this anomaly using its simulation. The unexpected beading of water on this class of surfaces can be explained by the rapid drop of the electric field in a dense lattice of C-F dipoles.
Intentionally adjusting wetting behavior on a surface
The scientists studied the binding of water to a fluorinated diamond surface with the help of multiscale simulation. In order to estimate the binding energy, they studied the adherence of individual water molecules on the surface as a first step using quantum-mechanical calculations of the electronic structure. “We also wanted to understand the effect at the fundamental level,” according to Moras. “With that as a starting point, we then scaled up the simulation to many water molecules so that the behavior of water drops can be mapped.” The insights from the multiscale model are far-reaching. “It becomes clear from our simulation that for a 100% fluorinated, extremely polarized surface, the electric dipole fields of the molecules are superposed in such a way that the electrostatic interaction falls off extremely rapidly, and the water is unable to adhere,” explains Mayrhofer. This rapid fall-off of the electric field had already been predicted by Lennard-Jones in 1928 for dense lattices of mathematical dipoles, but until now had not been associated with polar hydrophobicity. The scientists carried out the same simulation for a surface that was 50 percent fluorinated. This showed that the behavior of the water molecules changed depending on how densely the dipole lattice was packed with fluorine at the surface. “We are able to adjust the contact angle of the water drops in this way," explains Mayrhofer. The greater the contact angle is, the less the water adheres to the surface.
The simulation can be carried out for any surface and liquid
What is now crucial: this simulation method allows for the prediction of the wetting behavior of arbitrary surfaces/liquids combinations. The wetting of surfaces plays a role in many areas. Mayrhofer and his colleagues can describe the behavior of oils on engine parts just as easily as that of bacterially contaminated liquids on medical equipment. “The first step to application development is a better understanding of fundamentals. With the framework developed in this collaborative study, we are able to better understand how to control surface-liquid interactions,” says Dr. Rajagopalan from ExxonMobil, “and this knowledge can enable design of optimal surface chemistry for specific applications.”
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.




























































