Scientists discover new phenomenon in the field of gas-solid interactions
Prof. Dr. Stefan Kaskel and his team of scientists at the Institute of Inorganic Chemistry I at TU Dresden discovered in cooperation with the Helmholtz-Centre Berlin (HZB) and French researchers a new phenomenon in the field of gas-solid interactions: the so-called Negative Gas Adsorption (NGA).
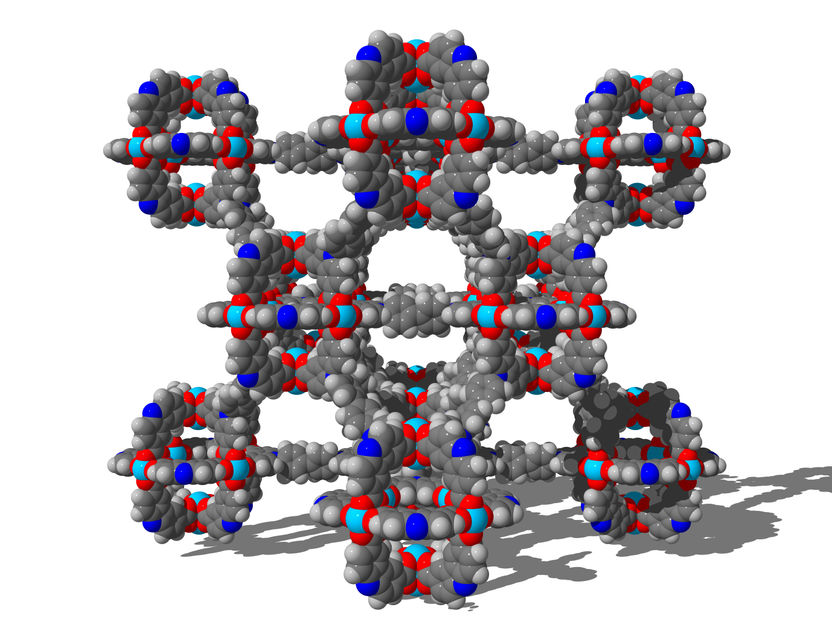
Network structure: Three-dimensional network structure of the highly porous and flexible material DUT-49 (DUT= Dresden University of Technology) which displays the NGA-effect
TU Dresden, Prof. AC1
Adsorption refers to the process by which molecules of a gas collect on the surface of a solid. Metal-organic frameworks are highly porous materials which are widely used for adsorptive applications such as in the reduction of pollutant emissions. The scientists from Dresden, Berlin and France have now succeeded in demonstrating that these materials can dynamically change their structures. During this change of structure, the scientists observed a so far unknown phenomenon: instead of absorbing the gas under pressure increase, the materials released the gas eruptively after reaching a certain threshold. This new phenomenon in the area of gas-solid interactions is therefore called Negative Gas Adsorption (NGA). Normally, materials respond to increased gas pressure by gathering molecules on the outer or inner surface and thus, the gas pressure is buffered and decreases with time. NGA materials can react towards pressure increase by releasing molecules. Hence, the pressure is further amplified. This counterintuitive phenomenon is triggered by solid state phase transitions. Similar to a volcano effect, a small trigger can cause a gas eruption out of this material. Gas pressure amplifying materials represent a new class of solids with potential applications in rescue systems, microengineering and separation applications.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.