Mechanism for radiation damage identified
Scientists examine electronic decay processes with the aid of quantum chemistry
What exactly are the processes when x-ray photons damage biomolecules with a metal centre? This question has been investigated by a team of scientists at the Institute for Physical Chemistry of Heidelberg University. Using the methods of quantum chemistry, they examined the underlying electronic processes caused by x-ray absorption. It turned out that the metal centre plays a key role in destroying a biomolecule.
radiation damage, arising from the interaction of high-energy X-rays with biological material, is a phenomenon widely known in science. It occurs, for example, when substrates – such as proteins – are analysed using x-ray light in order to determine their electronic structure or the spatial order of atoms. According to Prof. Dr. Lorenz S. Cederbaum, this damage is most visible in the direct neighbourhood of metal centres, which are essential for the stability and biological function of biomolecules.
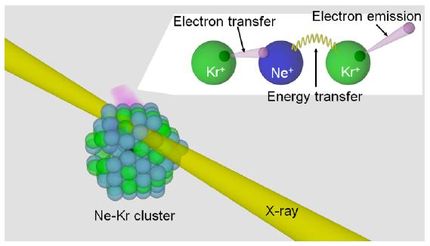
The Heidelberg researchers investigated the related processes of electronic decay using computer-aided methods from quantum chemistry. They focused on processes occurring when radiation is absorbed through the metal centre of a biomolecule. As a model system they used a microcluster. That is a chemical system in which water molecules are arranged around a metal centre, in this case the positive doubly charged magnesium ion.
Prof. Cederbaum explains that the metal centre initially loses several electrons through absorbing the radiation. That produces a highly charged, high-energy metal ion, which then returns to its original state through a cascade of electronic decay steps. In some of them the energy is transferred from the metal centre to the neighbouring molecules – a process known as Interatomic Coulombic decay (ICD). In others, electrons from the neighbouring molecules are transferred to the metal ion in the so called electron transfer mediated decay (ETMD).
According to Prof. Cederbaum, the two processes are ultrafast and take place on a scale of femtoseconds – the thousand millionth part of a micro-second. So they leave very little time for determination of the accurate molecular structure. In the course of the decay cascade, several neighbouring molecules emit slow electrons, both through the ICD and the ETMD processes. The molecules therefore charge positively, which leads to an explosion of the microcluster.
In a bigger system, e.g. a protein with a metal centre, the positively charged neighbouring molecules and the slow electrons would react with the biomolecule and do more secondary damage, Prof. Cederbaum adds. The metal centre works like a lens that focuses the energy of the x-ray light onto its immediate environment. The result is a massive alteration of the surrounding chemical structure on a fast time scale.
“We assume that the mechanism we have identified plays an important role when it comes to radiation damage in biological building-blocks with metal atoms – notably proteins and DNA,” says Prof. Cederbaum. He hopes that these findings will contribute to decoding the complicated processes caused by radiation in living organisms.
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents